Abbreviations Gly, G Molar mass 75.0666 g/mol Density 1.61 g/cm³ | Formula C2H5NO2 Melting point 233 °C Appearance white solid | |
 | ||
Thermodynamicdata Phase behavioursolid–liquid–gas | ||
The anti inflammatory properties of the amino acid glycine part 1
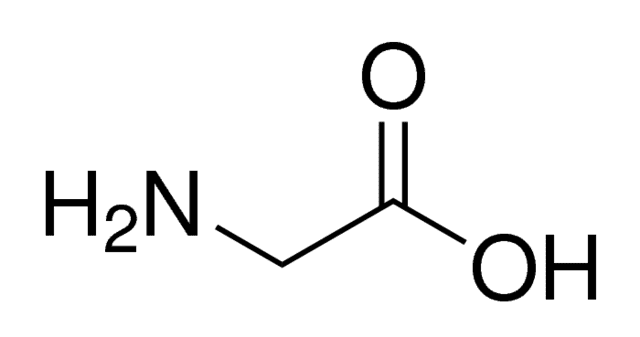
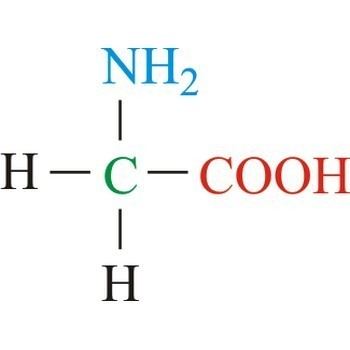
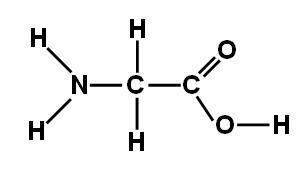
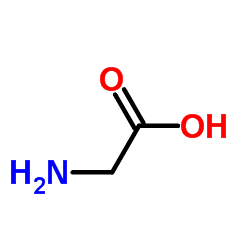
Glycine (abbreviated as Gly or G) is the amino acid that has a single hydrogen atom as its side chain. It is the simplest possible amino acid. The chemical formula of glycine is NH2‐CH2‐COOH. Glycine is one of the proteinogenic amino acids. Its codons are GGU, GGC, GGA, GGG of the genetic code.
Contents
- The anti inflammatory properties of the amino acid glycine part 1
- Glycine
- History and etymology
- Production
- Biosynthesis
- Degradation
- Physiological function
- As a biosynthetic intermediate
- As a neurotransmitter
- Uses
- Animal and human foods
- Cosmetics and miscellaneous applications
- Chemical feedstock
- Laboratory research
- Presence in space
- References

Glycine is a colorless, sweet-tasting crystalline solid. It is unique among the proteinogenic amino acids in that it is achiral. It can fit into hydrophilic or hydrophobic environments since it exists as zwitterion at natural pH, due to its minimal side chain of only one hydrogen atom. The acyl radical is glycyl.
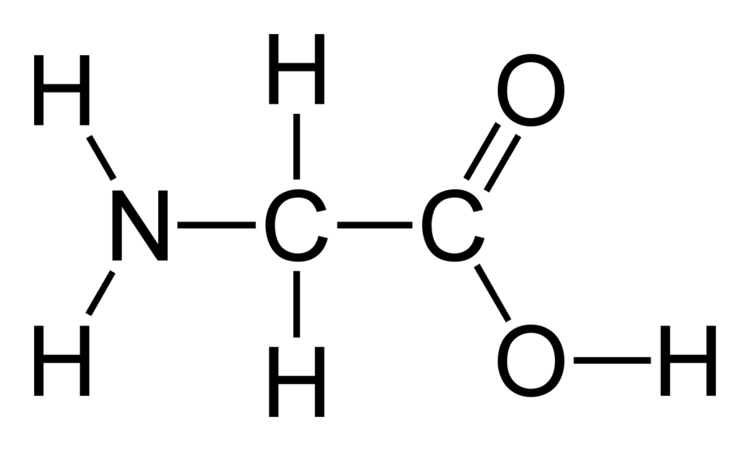
Glycine
History and etymology
Glycine was first isolated from gelatin in 1820. The name comes from the ancient Greek word γλυκύς "sweet tasting" (which is also related to the prefixes glyco- and gluco-, as in glycoprotein and glucose).
Production
Glycine was discovered in 1820, by Henri Braconnot who boiled a gelatinous object with sulfuric acid.
Glycine is manufactured industrially by treating chloroacetic acid with ammonia:
ClCH2COOH + 2 NH3 → H2NCH2COOH + NH4ClAbout 15 million kg are produced annually in this way.
In the USA (by GEO Specialty Chemicals, Inc.) and in Japan (by Showa Denko KK), glycine is produced via the Strecker amino acid synthesis.

There are two producers of glycine in the United States: Chattem Chemicals, Inc., a subsidiary of Mumbai-based Sun Pharmaceutical, and GEO Specialty Chemicals, Inc., which purchased the glycine and naphthalene sulfonate production facilities of Hampshire Chemical Corp, a subsidiary of Dow Chemical.
Chattem's manufacturing process ("MCA" process) occurs in batches and results in a finished product with some residual chloride but no sulfate, while GEO’s manufacturing process is considered a semi-batch process and results in a finished product with some residual sulfate but no chloride.
Glycine is also cogenerated as an impurity in the synthesis of EDTA, arising from reactions of the ammonia coproduct.
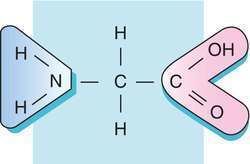
In aqueous solution, glycine itself is amphoteric: at low pH the molecule can be protonated with a pKa of about 2.4 and at high pH it loses a proton with a pKa of about 9.6 (precise values of pKa depend on temperature and ionic strength). The nature of glycine in aqueous solution has been investigated by theoretical methods. In solution the ratio of concentrations of the two isomers is independent of both the analytical concentration and of pH. This ratio is simply the equilibrium constant for isomerization.
K = [NH3+CH2CO−2]/[H2NCH2CO2H]
Both isomers of glycine have been observed by microwave spectroscopy in the gas phase. The solid-state structure has been analyzed in detail.
Biosynthesis
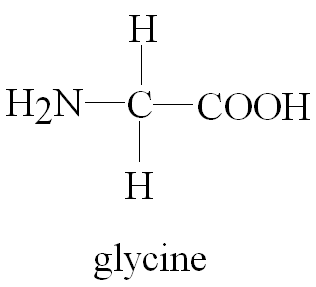
Glycine is not essential to the human diet, as it is biosynthesized in the body from the amino acid serine, which is in turn derived from 3-phosphoglycerate, but the metabolic capacity for glycine biosynthesis does not satisfy the need for collagen synthesis. In most organisms, the enzyme serine hydroxymethyltransferase catalyses this transformation via the cofactor pyridoxal phosphate:
serine + tetrahydrofolate → glycine + N5,N10-Methylene tetrahydrofolate + H2O
In the liver of vertebrates, glycine synthesis is catalyzed by glycine synthase (also called glycine cleavage enzyme). This conversion is readily reversible:
CO2 + NH+4 + N5,N10-Methylene tetrahydrofolate + NADH + H+ → Glycine + tetrahydrofolate + NAD+
Glycine is coded by codons GGU, GGC, GGA and GGG. Most proteins incorporate only small quantities of glycine. A notable exception is collagen, which contains about 35% glycine.
Degradation
Glycine is degraded via three pathways. The predominant pathway in animals and plants involves the glycine cleavage enzyme:
Glycine + tetrahydrofolate + NAD+ → CO2 + NH+4 + N5,N10-Methylene tetrahydrofolate + NADH + H+
In the second pathway, glycine is degraded in two steps. The first step is the reverse of glycine biosynthesis from serine with serine hydroxymethyl transferase. Serine is then converted to pyruvate by serine dehydratase.
In the third pathway of glycine degradation, glycine is converted to glyoxylate by D-amino acid oxidase. Glyoxylate is then oxidized by hepatic lactate dehydrogenase to oxalate in an NAD+-dependent reaction.
The half-life of glycine and its elimination from the body varies significantly based on dose. In one study, the half-life was between 0.5 and 4.0 hours.
Physiological function
The principal function of glycine is as a precursor to proteins, such as its periodically repeated role in the formation of the collagen helix in conjunction with hydroxyproline. It is also a building block for numerous natural products.
As a biosynthetic intermediate
In higher eukaryotes, δ-aminolevulinic acid, the key precursor to porphyrins, is biosynthesized from glycine and succinyl-CoA by the enzyme ALA synthase. Glycine provides the central C2N subunit of all purines.
As a neurotransmitter
Glycine is an inhibitory neurotransmitter in the central nervous system, especially in the spinal cord, brainstem, and retina. When glycine receptors are activated, chloride enters the neuron via ionotropic receptors, causing an Inhibitory postsynaptic potential (IPSP). Strychnine is a strong antagonist at ionotropic glycine receptors, whereas bicuculline is a weak one. Glycine is a required co-agonist along with glutamate for NMDA receptors. In contrast to the inhibitory role of glycine in the spinal cord, this behaviour is facilitated at the (NMDA) glutamatergic receptors which are excitatory. The LD50 of glycine is 7930 mg/kg in rats (oral), and it usually causes death by hyperexcitability.
A 2014 review on sleep aids noted that glycine can improve sleep quality, citing a study in which 3 grams of glycine before bedtime improved sleep quality in humans. Glycine has also been positively tested as an add-on treatment for schizophrenia.
Uses
In the US, glycine is typically sold in two grades: United States Pharmacopeia (“USP”), and technical grade. Most glycine is manufactured as USP grade material for diverse uses. USP grade sales account for approximately 80 to 85 percent of the U.S. market for glycine.
Animal and human foods
Other markets for USP grade glycine include its use an additive in pet food and animal feed. For humans, glycine is sold as a sweetener/taste enhancer. Certain food supplements and protein drinks contain glycine. Certain drug formulations include glycine to improve gastric absorption of the drug.
Cosmetics and miscellaneous applications
Glycine serves as a buffering agent in antacids, analgesics, antiperspirants, cosmetics, and toiletries.
Many miscellaneous products use glycine or its derivatives, such as the production of rubber sponge products, fertilizers, metal complexants.
Chemical feedstock
Glycine is an intermediate in the synthesis of a variety of chemical products. It is used in the manufacture of the herbicide glyphosate.
Laboratory research
Glycine is a significant component of some solutions used in the SDS-PAGE method of protein analysis. It serves as a buffering agent, maintaining pH and preventing sample damage during electrophoresis. Glycine is also used to remove protein-labeling antibodies from Western blot membranes to enable the probing of numerous proteins of interest from SDS-PAGE gel. This allows more data to be drawn from the same specimen, increasing the reliability of the data, reducing the amount of sample processing, and number of samples required. This process is known as stripping.
Presence in space
In 2009, glycine sampled in 2004 from comet Wild 2 by the NASA spacecraft Stardust was confirmed – the first discovery of glycine outside the Earth, although glycine had been identified in the Murchison meteorite in 1970. The discovery of cometary glycine bolstered the theory of panspermia, which claims that the "building-blocks" of life are widespread throughout the Universe. In 2016, detection of glycine within Comet 67P/Churyumov-Gerasimenko by the Rosetta spacecraft was announced.
The detection of glycine outside the solar system in the interstellar medium has been debated. In 2008, the glycine-like molecule aminoacetonitrile was discovered in the Large Molecule Heimat, a giant gas cloud near the galactic center in the constellation Sagittarius by the Max Planck Institute for Radio Astronomy.
