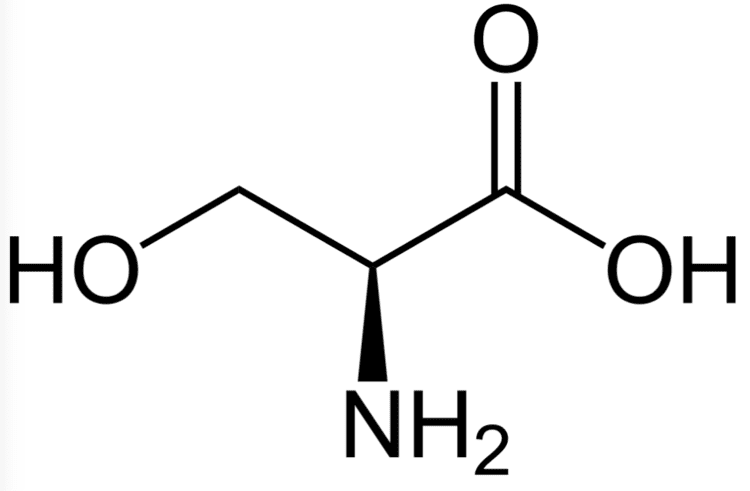
Formula C3H7NO3 Classification Pacifastin | Density 1.6 g/cm³ Appearance white crystals or powder | |
 | ||
Thermodynamicdata Phase behavioursolid–liquid–gas | ||
Serine synthesis
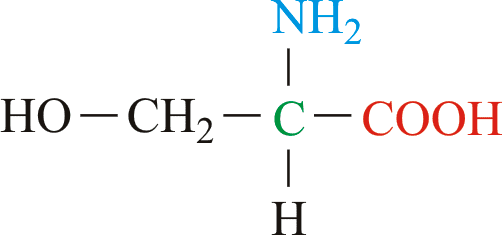
Serine (abbreviated as Ser or S) encoded by the codons UCU, UCC, UCA, UCG, AGU and AGC is an ɑ-amino acid that is used in the biosynthesis of proteins. It contains an α-amino group (which is in the protonated −NH+
3 form under biological conditions), a carboxyl group (which is in the deprotonated –COO−
form in physiological conditions), and a side chain consisting of a hydroxymethyl group (see hydroxyl), classifying it as a polar amino acid. It can be synthesized in the human body under normal physiological circumstances, making it a nonessential amino acid.
Contents
- Serine synthesis
- Occurrence
- Biosynthesis
- Synthesis and industrial production
- Metabolic
- Structural role
- Signaling
- Gustatory sensation
- Clinical significance
- Research for therapeutic use
- References
Occurrence
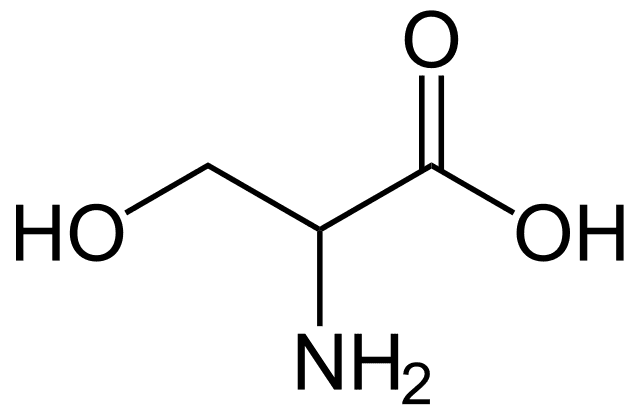
This compound is one of the naturally occurring proteinogenic amino acids. Only the L-stereoisomer appears naturally in proteins. It is not essential to the human diet, since it is synthesized in the body from other metabolites, including glycine. Serine was first obtained from silk protein, a particularly rich source, in 1865. Its name is derived from the Latin for silk, sericum. Serine's structure was established in 1902.
Biosynthesis
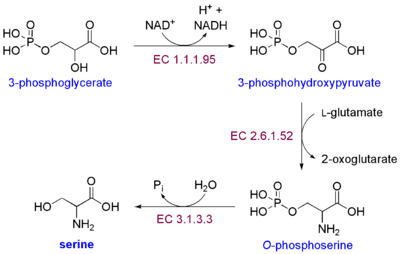
The biosynthesis of serine starts with the oxidation of 3-phosphoglycerate (an intermediate from glycolysis) to 3-phosphohydroxypyruvate and NADH by phosphoglycerate dehydrogenase (EC 1.1.1.95). Reductive amination (transamination) of this ketone by phosphoserine transaminase (EC 2.6.1.52) yields 3-phosphoserine (O-phosphoserine) which is hydrolyzed to serine by phosphoserine phosphatase (EC 3.1.3.3).
In bacteria such as E. coli these enzymes are encoded by the genes serA (EC 1.1.1.95), serC (EC 2.6.1.52), and serB (EC 3.1.3.3).
Glycine biosynthesis: Serine hydroxymethyltransferase (SHMT = serine transhydroxymethylase) also catalyzes the reversible conversions of L-serine to glycine (retro-aldol cleavage) and 5,6,7,8-tetrahydrofolate to 5,10-methylenetetrahydrofolate (mTHF) (hydrolysis). SHMT is a pyridoxal phosphate (PLP) dependent enzyme. Glycine can also be formed from CO2, NH4+, and mTHF in a reaction catalyzed by glycine synthase.
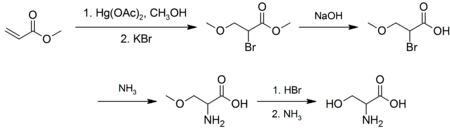
Synthesis and industrial production
Industrially, L-serine is produced by fermentation, with an estimated 100-1000 tonnes per year produced. In the laboratory, racemic serine can be prepared from methyl acrylate via several steps:
Metabolic
Serine is important in metabolism in that it participates in the biosynthesis of purines and pyrimidines. It is the precursor to several amino acids including glycine and cysteine, and tryptophan in bacteria. It is also the precursor to numerous other metabolites, including sphingolipids and folate, which is the principal donor of one-carbon fragments in biosynthesis.
Structural role
Serine plays an important role in the catalytic function of many enzymes. It has been shown to occur in the active sites of chymotrypsin, trypsin, and many other enzymes. The so-called nerve gases and many substances used in insecticides have been shown to act by combining with a residue of serine in the active site of acetylcholine esterase, inhibiting the enzyme completely.
Serine sidechains are often hydrogen bonded; the commonest small motifs formed are ST turns, ST motifs (often at the beginning of alpha helices) and ST staples (usually at the middle of alpha helices).
As a constituent (residue) of proteins, its side chain can undergo O-linked glycosylation, which may be functionally related to diabetes.
It is one of three amino acid residues that are commonly phosphorylated by kinases during cell signaling in eukaryotes. Phosphorylated serine residues are often referred to as phosphoserine.
Serine proteases are a common type of protease.
Signaling
D-Serine, synthesized in the brain by serine racemase from L-serine (its enantiomer), serves as a neuromodulator by coactivating NMDA receptors, making them able to open if they then also bind glutamate. D-serine is a potent agonist at the glycine site of the NMDA-type glutamate receptor (NMDAR). For the receptor to open, glutamate and either glycine or D-serine must bind to it; in addition a pore blocker must not be bound (e.g. Mg2+ or Pb2+). In fact, D-serine is a more potent agonist at the glycine site on the NMDAR than glycine itself.
D-serine was thought to exist only in bacteria until relatively recently; it was the second D amino acid discovered to naturally exist in humans, present as a signalling molecule in the brain, soon after the discovery of D-aspartate. Had D amino acids been discovered in humans sooner, the glycine site on the NMDA receptor might instead be named the D-serine site. Apart from central nervous system, D-serine plays a signaling role in peripheral tissues and organs such as cartilage, kidney and corpus cavernosum.
Gustatory sensation
L-Serine is sweet with minor umami and sour tastes at high concentration.
Pure D-serine is an off-white crystalline powder with a very faint musty aroma. D-Serine is sweet with an additional minor sour taste at medium and high concentrations.
Clinical significance
Serine deficiency disorders are rare defects in the biosynthesis of the amino acid L-serine. At present three disorders have been reported: 3-phosphoglycerate dehydrogenase deficiency, 3-phosphoserine phosphatase deficiency and Phosphoserine aminotransferase deficiency. These enzyme defects lead to severe neurological symptoms such as congenital microcephaly and severe psychomotor retardation and in addition in patients with 3-phosphoglycerate dehydrogenase deficiency to intractable seizures. These symptoms respond to a variable degree to treatment with L-serine, sometimes combined with glycine. Response to treatment is variable and the long-term and functional outcome is unknown. To provide a basis for improving the understanding of the epidemiology, genotype/phenotype correlation and outcome of these diseases their impact on the quality of life of patients, and for evaluating diagnostic and therapeutic strategies a patient registry was established by the noncommercial International Working Group on Neurotransmitter Related Disorders (iNTD).
Research for therapeutic use
D-Serine is being studied in rodents as a potential treatment for schizophrenia and L-serine is in FDA-approved human clinical trials as a possible treatment for ALS. A 2011 meta-analysis found adjunctive sarcosine to have a medium effect size for negative and total symptoms. D-Serine has also been described as a potential biomarker for early Alzheimer's disease (AD) diagnosis, due to a relatively high concentration of it in the cerebrospinal fluid of probable AD patients.
