 | ||
Sperm guidance is the process by which sperm cells (spermatozoa) are directed to the oocyte (egg) for the aim of fertilization. In the case of marine invertebrates the guidance is done by chemotaxis. In the case of mammals, it appears to be done by chemotaxis, thermotaxis and rheotaxis.
Contents
- Background
- Sperm guidance in non mammalian species
- Chemoattractants
- Table 1 Some sperm chemoattractants in non mammalian species
- Species specificity
- Behavioral mechanism
- Molecular mechanism
- Sperm guidance in mammals I Chemotaxis
- Sperm guidance in mammals II Thermotaxis
- Sperm guidance in mammals III Chemotaxis and thermotaxis combined
- Potential clinical applications
- References
Background
Since the discovery of sperm attraction to the female gametes in ferns over a century ago, sperm guidance in the form of sperm chemotaxis has been established in a large variety of species Although sperm chemotaxis is prevalent throughout the Metazoa kingdom, from marine species with external fertilization such as sea urchins and corals, to humans, much of the current information on sperm chemotaxis is derived from studies of marine invertebrates, primarily sea urchin and starfish. As a matter of fact, until not too long ago, the dogma was that, in mammals, guidance of spermatozoa to the oocyte was unnecessary. This was due to the common belief that, following ejaculation into the female genital tract, large numbers of spermatozoa 'race' towards the oocyte and compete to fertilize it. This belief was taken apart when it became clear that only few of the ejaculated spermatozoa — in humans, only ~1 of every million spermatozoa — succeed in entering the oviducts (Fallopian tubes) and when more recent studies showed that mammalian spermatozoa employ at least three different mechanisms, each of which can potentially serve as a guidance mechanism: chemotaxis, thermotaxis and rheotaxis.
Sperm guidance in non-mammalian species
Sperm guidance in non-mammalian species is performed by chemotaxis. The oocyte secretes a chemoattractant, which, as it diffuses away, forms a concentration gradient: a high concentration close to the egg, and a gradually lower concentration as the distance from the oocyte increases. Spermatozoa can sense this chemoattractant and orient their swimming direction up the concentration gradient towards the oocyte. Sperm chemotaxis was demonstrated in a large number of non-mammalian species, from marine invertebrates to frogs.
Chemoattractants
The sperm chemoattractants in non-mammalian species vary to a large extent. Some examples are shown in Table 1. So far, most sperm chemoattractants that have been identified in non-mammalian species are peptides or low-molecular-weight proteins (1–20 kDa), which are heat stable and sensitive to proteases. Exceptions to this rule are the sperm chemoattractants of corals, ascidians, plants such as ferns, and algae (Table 1).
Table 1. Some sperm chemoattractants in non-mammalian species*
Species specificity
The variety of chemoattractants raises the question of species specificity with respect to the chemoattractant identity. There is no single rule for chemoattractant-related specificity. Thus, in some groups of marine invertebrates (e.g., hydromedusae and certain ophiuroids), the specificity is very high; in others (e.g., starfish), the specificity is at the family level and, within the family, there is no specificity. In mollusks, there appears to be no specificity at all. Likewise, in plants, a unique simple compound [e.g., fucoserratene — a linear, unsaturated alkene (1,3-trans 5-cis-octatriene)] might be a chemoattractant for various species.
Behavioral mechanism
Here, too, there is no single rule. In some species (for example, in hydroids like Campanularia or tunicate like Ciona), the swimming direction of the spermatozoa changes abruptly towards the chemoattractant source. In others (for example, in sea urchin, hydromedusa, fern, or fish such as Japanese bitterlings), the approach to the chemoattractant source is indirect and the movement is by repetitive loops of small radii. In some species (for example, herring or the ascidian Ciona) activation of motility precedes chemotaxis. In chemotaxis, cells may either sense a temporal gradient of the chemoattractant, comparing the occupancy of its receptors at different time points (as do bacteria), or they may detect a spatial gradient, comparing the occupancy of receptors at different locations along the cell (as do leukocytes). In the best-studied species, sea urchin, the spermatozoa sense a temporal gradient and respond to it with a transient increase in flagellar asymmetry. The outcome is a turn in the swimming path, followed by a period of straight swimming, leading to the observed epicycloid-like movements directed towards the chemoattractant source.
Molecular mechanism
The molecular mechanism of sperm chemotaxis is still not fully known. The current knowledge is mainly based on studies in the sea urchin Arbacia punctulata, where binding of the chemoattractant resact (Table 1) to its receptor, a guanylyl cyclase, activates cGMP synthesis (Figure 1). The resulting rise of cGMP possibly activates K+-selective ion channels. The consequential hyperpolarization activates hyperpolarization-activated and cyclic nucleotide-gated (HCN) channels. The depolarizing inward current through HCN channels possibly activates voltage-activated Ca2+ channels, resulting in elevation of intracellular Ca2+. This rise leads to flagellar asymmetry and, consequently, a turn of the sperm cell.
Figure 1. A model of the signal-transduction pathway during sperm chemotaxis of the sea urchin Arbacia punctulata. Binding of a chemoattractant (ligand) to the receptor — a membrane-bound guanylyl cyclase (GC) — activates the synthesis of cGMP from GTP. Cyclic GMP possibly opens cyclic nucleotide-gated (CNG) K+-selective channels, thereby causing hyperpolarization of the membrane. The cGMP signal is terminated by the hydrolysis of cGMP through phosphodiesterase (PDE) activity and inactivation of GC. On hyperpolarization, hyperpolarization-activated and cyclic nucleotide-gated (HCN) channels allow the influx of Na+ that leads to depolarization and thereby causes a rapid Ca2+ entry through voltage-activated Ca2+ channels (Cav), Ca2+ ions interact by unknown mechanisms with the axoneme of the flagellum and cause an increase of the asymmetry of flagellar beat and eventually a turn or bend in the swimming trajectory. Ca2+ is removed from the flagellum by a Na+/Ca2+ exchange mechanism. [Taken from ref.]
Sperm guidance in mammals: I. Chemotaxis
Following the findings that human spermatozoa accumulate in follicular fluid and that there is a remarkable correlation between this in vitro accumulation and oocyte fertilization, chemotaxis was substantiated as the cause of this accumulation. Sperm chemotaxis was later also demonstrated in mice and rabbits. In addition, sperm accumulation in follicular fluid (but without substantiating that it truly reflects chemotaxis) was demonstrated in horses and pigs. A key feature of sperm chemotaxis in humans is that this process is restricted to capacitated cells — the only cells that possess the ability to penetrate the oocyte and fertilize it. This raised the possibility that, in mammals, chemotaxis is not solely a guidance mechanism but it is also a mechanism of sperm selection. Importantly, the fraction of capacitated (and, hence, chemotactically responsive) spermatozoa is low (~10% in humans), the life span of the capacitated/chemotactic state is short (1–4 hours in humans), a spermatozoon can be at this state only once in its lifetime, and sperm individuals become capacitated/chemotactic at different time points, resulting in continuous replacement of capacitated/chemotactic cells within the sperm population, i.e., prolonged availability of capacitated cells. These sperm features raised the possibility that prolonging the time period, during which capacitated spermatozoa can be found in the female genital tract, is a mechanism, evolved in humans, to compensate for the lack of coordination between insemination and ovulation.
Chemoattractants
In humans, there are at least two different origins of sperm chemoattractants. One is the cumulus cells that surround the oocyte, and the other is the mature oocyte itself. The chemoattractant secreted from the cumulus cells is the steroid progesterone, shown to be effective at the picomolar range. The chemoattractant secreted from the oocyte is even more potent. It is a hydrophobic non-peptide molecule which, when secreted from the oocyte, is in complex with a carrier protein Additional compounds have been shown to act as chemoattractants for mammalian spermatozoa. They include the chemokine CCL20, atrial natriuretic peptide (ANP), specific odorants, and allurin, to mention a few. It is reasonable to assume that not all of them are physiologically relevant.
Species specificity
Species specificity was not detected in experiments that compared the chemotactic responsiveness of human and rabbit spermatozoa to follicular fluids or egg-conditioned media obtained from human, bovine, and rabbit. The subsequent findings that cumulus cells of both human and rabbit (and, probably, of other mammals as well) secrete the chemoattractant progesterone is sufficient to account for the lack of specificity in the chemotactic response of mammalian spermatozoa.
Behavioral mechanism
Mammalian spermatozoa, like sea-urchin spermatozoa, appear to sense the chemoattractant gradient temporally (comparing receptor occupancy over time) rather than spatially (comparing receptor occupancy over space). This is because the establishment of a temporal gradient in the absence of spatial gradient, achieved by mixing human spermatozoa with a chemoattractant or by photorelease of a chemoattractant from its caged compound, results in delayed transient changes in swimming behavior that involve increased frequency of turns and hyperactivation events. On the basis of these observations and the finding that the level of hyperactivation events is reduced when chemotactically responsive spermatozoa swim in a spatial chemoattractant gradient it was proposed that turns and hyperactivation events are suppressed when capacitated spermatozoa swim up a chemoattractant gradient, and vice versa when they swim down a gradient.
Molecular mechanism
Very little is known on the molecular mechanism of sperm chemotaxis in mammals. It is even not known whether it is similar to that of sea-urchin spermatozoa because, on the one hand, assuming universality — the molecular mechanisms may be similar. The finding in human spermatozoa that ANP, a known activator of particulate guanylyl cyclase, is chemotactically active and the consequent suggestion that ANP may directly affect guanylyl cyclase in a manner similar to that caused by the physiological attractant (Zamir et al., 1993) are in line with this possibility. On the other hand, the identification of the odorant receptor hOR17-4 on human spermatozoa and the demonstration of sperm chemotaxis to its agonist bourgeonal (Spehr et al., 2003) suggest that mammalian sperm chemotaxis involves a signal transduction pathway similar to that of the olfactory system. This may seem a valid possibility in view of the finding that male germ cells appear to contain all the elements of the signaling cascade present in olfactory cells (Defer et al., 1998) and the observation that bourgeonal induces a transient Ca2+ influx in about one third of the cells of human spermatozoa, a response that is inhibited by an adenylyl cyclase inhibitor (Spehr et al., 2003; Spehr et al., 2004). It is possible that mammalian spermatozoa possess both signal-transduction systems. The observation that intracellular photorelease of each cAMP and cGMP causes a similar behavioral response (Gakamsky et al., 2009) is consistent with this possibility.
Sperm guidance in mammals: II. Thermotaxis
At ovulation, at least in rabbits (Bahat et al., 2005; David et al., 1972) and pigs (Hunter and Nichol, 1986), a temperature difference of 1–2 °C is established within the oviduct, the temperature being higher at the fertilization site than at the junction between the uterus and the oviduct, close to the sperm storage site in the oviduct (Suarez, 2002). This difference is formed by a time-dependent temperature drop at the uterus-oviduct junction, a drop that occurs in spite of the simultaneous rise in body temperature at ovulation (Bahat et al., 2005). Following the suggestion of Hunter (1998) that this temperature difference might serve as a cue for guiding spermatozoa to the site of fertilization, Bahat et al. (2003) demonstrated that rabbit and human spermatozoa are able to sense small temperature differences and respond to them by thermotaxis. The temperature gradient in the rabbit oviduct was calculated to be at the order of 0.1 °C/cm (Bahat and Eisenbach, 2006), within the range of known thermotaxis systems. As in sperm chemotaxis, only capacitated spermatozoa are thermotactically responsive (Bahat et al., 2003).
Sperm guidance in mammals: III. Chemotaxis and thermotaxis combined
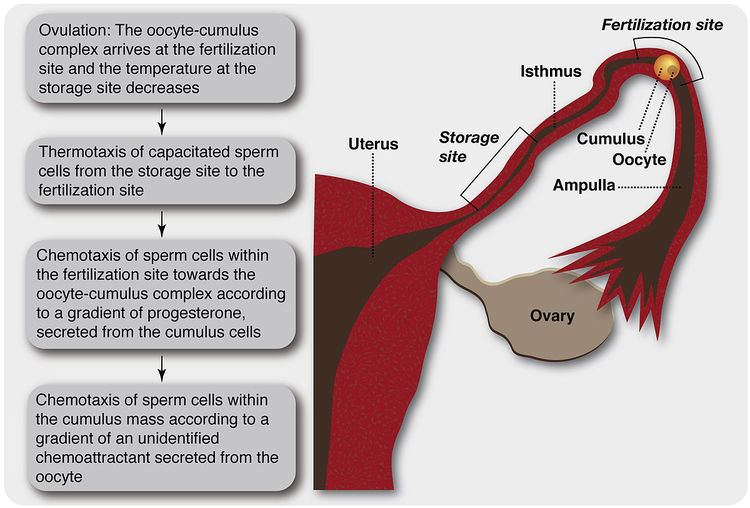
The findings that mammalian spermatozoa are capable to respond to a chemoattractant gradient by chemotaxis and to a temperature gradient by thermotaxis and that both such gradients are established in the female genital tract raised the possibility that spermatozoa guidance is done by both chemotaxis and thermotaxis. It was further suggested that, in vivo, each mechanism is functional in a region of the oviduct where the other mechanism is ineffective (Bahat et al., 2003; Eisenbach and Giojalas, 2006). According to this suggestion (Figure 2), capacitated spermatozoa, released from the sperm storage site at the isthmus (Suarez, 2002), may be first guided by thermotaxis from the cooler sperm storage site towards the warmer fertilization site (Bahat et al., 2003). Passive contractions of the oviduct (Battalia and Yanagimachi, 1979) may assist the spermatozoa to reach there. At this location the spermatozoa may be chemotactically guided to the oocyte-cumulus complex by the gradient of progesterone, secreted from the cumulus cells. In addition, progesterone may inwardly guide spermatozoa, already present within the periphery of the cumulus oophorus (Teves et al., 2006). Spermatozoa that are already deep within the cumulus oophorus may sense the more potent chemoattractant that is secreted from the oocyte (Sun et al., 2005) and chemotactically guide themselves to the oocyte according to the gradient of this chemoattractant. It should be borne in mind, however, that this is only a model. In view of the increasing number of different chemoattractants that are being discovered, the guidance in vivo might be much more complex.
Figure 2. A simplified scheme describing the suggested sequence of sperm guidance events in mammals.
Potential clinical applications
Sperm guidance by either chemotaxis or thermotaxis can potentially be used to obtain sperm populations that are enriched with capacitated spermatozoa for in vitro fertilization procedures. They can also be exploited as a diagnostic tool to assess sperm quality. In addition, these processes can potentially be used, in the long run, as a means of contraception by interfering with the normal process of fertilization (Eisenbach and Tur-Kaspa, 1999; Eisenbach and Giojalas, 2006).
