Abbreviations Met, M Molar mass 149.21 g/mol Density 1.34 g/cm³ Appearance White crystalline powder | Formula C5H11NO2S Melting point 281 °C Soluble in Water | |
 | ||
Thermodynamicdata Phase behavioursolid–liquid–gas IUPAC ID 2-amino-4-(methylthio)butanoic acid | ||
Methionine is an essential amino acid in humans. Methionine is important in angiogenesis, the growth of new blood vessels, and supplementation may benefit those suffering from Parkinson's, drug withdrawal, schizophrenia, radiation, copper poisoning, asthma, allergies, alcoholism, or depression. Overconsumption of methionine, as is typical in the standard American diet, but not vegan diet (although nuts, soy, and beans also have high levels of methionine), is related to cancer growth in a number of studies.
Contents
- Bio medical details
- A proteinogenic amino acid
- Encoding
- S adenosyl methionine
- Biosynthesis
- Trans sulfurylation pathway
- Other biochemical pathways
- Catabolism
- Regeneration
- Reverse transulfurylation pathway conversion to cysteine
- Ethylene synthesis
- Chemical synthesis
- Dietary sources
- Restriction
- Health
- Other uses
- References
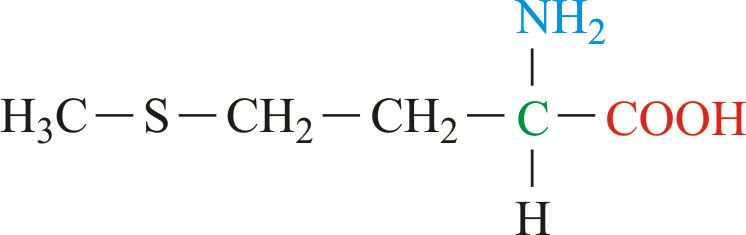
Methionine was first isolated in 1921 by John Howard Mueller.
Bio-medical details
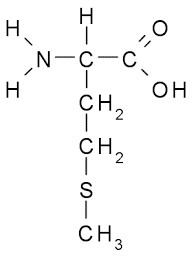
Methionine (abbreviated as Met or M; encoded by the codon AUG) is an α-amino acid that is used in the biosynthesis of proteins. It contains an α-amino group (which is in the protonated −NH3+ form under biological conditions), an α-carboxylic acid group (which is in the deprotonated −COO− form under biological conditions), and an S-methyl thioether side chain, classifying it as a non-polar, aliphatic amino acid.
Methionine is coded for by the initiation codon, meaning it indicates the start of the coding region and is the first amino acid produced in a nascent polypeptide during mRNA translation.
A proteinogenic amino acid

Together with cysteine, methionine is one of two sulfur-containing proteinogenic amino acids. Excluding the few exceptions where methionine may act as a redox sensor (e.g. ), methionine residues do not have a catalytic role. This is in contrast to cysteine residues, where the thiol group has a catalytic role in many proteins. The thioether does however have a minor structural role due to the stability effect of S/π interactions between the side chain sulfur atom and aromatic amino acids in one-third of all known protein structures. This lack of a strong role is reflected in experiments where little effect is seen in proteins where methionine is replaced by norleucine, a straight hydrocarbon sidechain amino acid which lacks the thioether. It has been conjectured that norleucine was present in early versions of the genetic code, but methionine intruded into the final version of the genetic code due to the fact it is used in the cofactor S-adenosyl methionine (SAM). This situation is not unique and may have occurred with ornithine and arginine.
Encoding
Methionine is one of only two amino acids encoded by a single codon (AUG) in the standard genetic code (tryptophan, encoded by UGG, is the other). In reflection to the evolutionary origin of its codon, the other AUN codons encode isoleucine, which is also a hydrophobic amino acid. In the mitochondrial genome of several organisms, including metazoa and yeast, the codon AUA also encodes for methionine. In the standard genetic code AUA codes for isoleucine and the respective tRNA (ileX in Escherichia coli) uses the unusual base lysidine (bacteria) or agmatine (archaea) to discriminate against AUG.
The methionine codon AUG is also the most common start codon. A "Start" codon is message for a ribosome that signals the initiation of protein translation from mRNA when the AUG codon is in a Kozak consensus sequence. As a consequence, methionine is often incorporated into the N-terminal position of proteins in eukaryotes and archaea during translation, although it can be removed by post-translational modification. In bacteria, the derivative N-formylmethionine is used as the initial amino acid.
S-adenosyl-methionine
The methionine-derivative S-adenosyl methionine (SAM) is a cofactor that serves mainly as a methyl donor. SAM is composed of an adenosyl molecule (via 5' carbon) attached to the sulfur of methionine, therefore making it a sulfonium cation (i.e. three substituents and positive charge). The sulfur acts as soft Lewis acid (i.e. donor/electrophile) allows the S-methyl group to be transferred to an oxygen, nitrogen or aromatic system, often with the aid of other cofactors such as cobalamin (vitamin B12 in humans). Some enzymes use SAM to initiate a radical reaction, these are called radical SAM enzymes. As a result of the transfer of the methyl group, S-adenosyl-homocysteine is obtained. In bacteria, this is either regenerated by methylation or is salvaged by removing the adenine and the homocysteine leaving the compound dihydroxypentandione to spontaneously convert into autoinducer-2, which is excreted as a waste product / quorum signal.
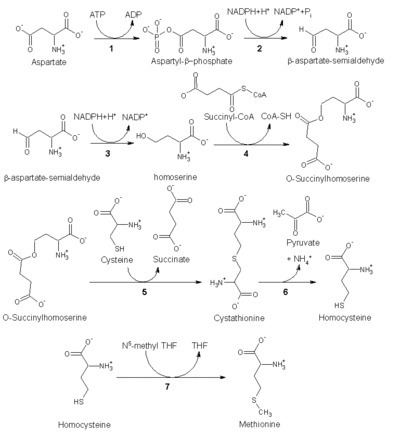
Biosynthesis
As an essential amino acid, methionine is not synthesized de novo in humans and other animals, who must ingest methionine or methionine-containing proteins. In plants and microorganisms, methionine biosynthesis belongs to the aspartate family, along with threonine and lysine (via diaminopimelate, but not via α-aminoadipate). The main backbone is derived from aspartic acid, while the sulfur may come from cysteine, methanethiol or hydrogen sulfide.
The pathway utilising cysteine is called the "transsulfuration pathway", while the pathway utilising hydrogen sulfide (or methanethiol) is called "direct-sulfurylation pathway".
Cysteine is similarly produced, namely it can be made from an activated serine and either from homocysteine ("reverse trans-sulfurylation route") or from hydrogen sulfide ("direct sulfurylation route"); the activated serine is generally O-acetyl-serine (via CysK or CysM in E. coli), but in Aeropyrum pernix and some other archaea O-phosphoserine is used. CysK and CysM are homologues, but belong to the PLP fold type III clade.
Trans-sulfurylation pathway
Enzymes involved in the E. coli trans-sulfurylation route of methionine biosynthesis:
- Aspartokinase
- Aspartate-semialdehyde dehydrogenase
- Homoserine dehydrogenase
- Homoserine O-transsuccinylase
- Cystathionine-γ-synthase
- Cystathionine-β-lyase
- Methionine synthase (in mammals, this step is performed by homocysteine methyltransferase or betaine—homocysteine S-methyltransferase.)
Other biochemical pathways
Although mammals cannot synthesize methionine, they can still use it in a variety of biochemical pathways:
Catabolism
Methionine is converted to S-adenosylmethionine (SAM) by (1) methionine adenosyltransferase.
SAM serves as a methyl-donor in many (2) methyltransferase reactions, and is converted to S-adenosylhomocysteine (SAH).
(3) Adenosylhomocysteinase converts SAH to homocysteine.
There are two fates of homocysteine: it can be used to regenerate methionine, or to form cysteine.
Regeneration
Methionine can be regenerated from homocysteine via (4) methionine synthase in a reaction that requires Vitamin B12 as a cofactor.
Homocysteine can also be remethylated using glycine betaine (NNN-trimethyl glycine, TMG) to methionine via the enzyme betaine-homocysteine methyltransferase (E.C.2.1.1.5, BHMT). BHMT makes up to 1.5% of all the soluble protein of the liver, and recent evidence suggests that it may have a greater influence on methionine and homocysteine homeostasis than methionine synthase.
Reverse-transulfurylation pathway: conversion to cysteine
Homocysteine can be converted to cysteine.
Ethylene synthesis
This amino acid is also used by plants for synthesis of ethylene. The process is known as the Yang Cycle or the methionine cycle.
Chemical synthesis
Racemic methionine can be synthesized from diethyl sodium phthalimidomalonate by alkylation with chloroethylmethylsulfide (ClCH2CH2SCH3) followed by hydrolysis and decarboxylation.
Dietary sources
High levels of methionine can be found in eggs, sesame seeds, Brazil nuts, fish, meats and some other plant seeds; methionine is also found in cereal grains. Most fruits and vegetables contain very little of it. Most legumes are also low in methionine. However, it is the combination of methionine and cystine which is considered for completeness of a protein. Racemic methionine is sometimes added as an ingredient to pet foods.
Restriction
There is scientific evidence that restricting methionine consumption can increase lifespans in some animals.
A 2005 study showed methionine restriction without energy restriction extends mouse lifespan.
A study published in Nature showed adding just the essential amino acid methionine to the diet of fruit flies under dietary restriction, including restriction of essential amino acids (EAAs), restored fertility without reducing the longer lifespans that are typical of dietary restriction, leading the researchers to determine that methionine “acts in combination with one or more other EAAs to shorten lifespan.”
Several studies showed that methionine restriction also inhibits aging-related disease processes in mice and inhibits colon carcinogenesis in rats. In humans, methionine restriction through dietary modification could be achieved through a vegan diet. Veganism being a completely plant based diet is typically very low in methionine, however certain nuts and legumes may provide higher levels.
A 2009 study on rats showed "methionine supplementation in the diet specifically increases mitochondrial ROS production and mitochondrial DNA oxidative damage in rat liver mitochondria offering a plausible mechanism for its hepatotoxicity".
However, since methionine is an essential amino acid, it cannot be entirely removed from animals' diets without disease or death occurring over time. For example, rats fed a diet without methionine developed steatohepatitis (fatty liver), anemia and lost two thirds of their body weight over 5 weeks. Administration of methionine ameliorated the pathological consequences of methionine deprivation.
Methionine might also be essential to reversing damaging methylation of glucocorticoid receptors caused by repeated stress exposures, with implications for depression.
Health
Loss of methionine has been linked to senile greying of hair. Its lack leads to a buildup of hydrogen peroxide in hair follicles, a reduction in tyrosinase effectiveness, and a gradual loss of hair color.
Methionine is an intermediate in the biosynthesis of cysteine, carnitine, taurine, lecithin, phosphatidylcholine, and other phospholipids. Improper conversion of methionine can lead to atherosclerosis.
Other uses
DL-Methionine is sometimes given as a supplement to dogs; It helps to reduce the chances of stones in dogs. Methionine is also known to increase the urinary excretion of quinidine by acidifying the urine. Aminoglycoside antibiotics used to treat urinary tract infections work best in alkaline conditions, and urinary acidification from using methionine can reduce its effectiveness. If a dog is on a diet that acidifies the urine, methionine should not be used.
Methionine is allowed as a supplement to organic poultry feed under the US certified organic program.
