Domain Eukaryota Rank Phylum | Scientific name Dinoflagellata Higher classification Alveolate | |
 | ||
Phylum Dinoflagellata; Bütschli 1885, Dinoflagellata; Bütschli 1885, sensu Gomez 2012 Lower classifications Similar Angomonas deanei, Chloroplast, Apicoplast | ||
The dinoflagellates (Greek δῖνος dinos "whirling" and Latin flagellum "whip, scourge") are a large group of flagellate eukaryotes that constitute the phylum Dinoflagellata. Most are marine plankton, but they are common in freshwater habitats, as well. Their populations are distributed depending on temperature, salinity, or depth. Many dinoflagellates are known to be photosynthetic, but a large fraction of these are in fact mixotrophic, combining photosynthesis with ingestion of prey (phagotrophy). In terms of number of species, dinoflagellates form one of the largest groups of marine eukaryotes, although this group is substantially smaller than the diatoms. Some species are endosymbionts of marine animals and play an important part in the biology of coral reefs. Other dinoflagellates are unpigmented predators on other protozoa, and a few forms are parasitic (see for example Oodinium, Pfiesteria). Some dinoflagellates produce resting stages, called dinoflagellate cysts or dinocysts, as part of their lifecycles.
Contents
- Dinoflagellates general description
- History
- Classification
- Identification
- Morphology
- Endosymbionts
- Theca structure and formation
- Habitats
- Nutritional strategies
- The dinoflagellate nucleus dinokaryon
- Lifecycle
- Harmful algal blooms
- Bioluminescence
- Lipid and sterol production
- Transport
- Genomics
- Evolutionary history
- Examples
- References
Dinoflagellates are considered to be protists, with their own division, Dinoflagellata.
About 1,555 species of free-living marine dinoflagellates are currently described. Another estimate suggests about 2,000 living species, of which more than 1,700 are marine (free-living, as well as benthic) and about 220 are from fresh water. The latest estimates suggest a total of 2,294 living dinoflagellate species, which includes marine, freshwater and parasitic dinoflagellates.
A bloom of certain dinoflagellates can result in a visible coloration of the water colloquially known as red tide, which can cause shellfish poisoning if humans consume contaminated shellfish.
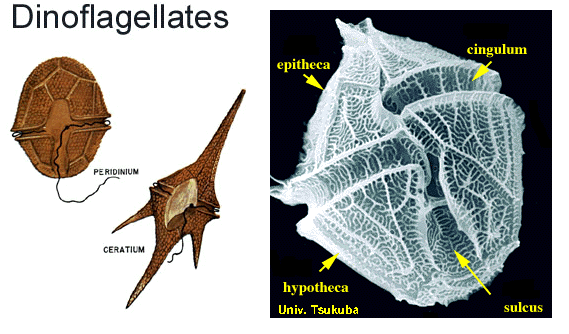
Dinoflagellates general description
History
In 1753, the first modern dinoflagellates were described by Henry Baker as "Animalcules which cause the Sparkling Light in Sea Water", and named by Otto Friedrich Müller in 1773. The term derives from the Greek word δῖνος (dinos), meaning whirling, and Latin flagellum, a diminutive term for a whip or scourge.
In the 1830s, the German microscopist Christian Gottfried Ehrenberg examined many water and plankton samples and proposed several dinoflagellate genera that are still used today including Peridinium, Prorocentrum, and Dinophysis.
These same dinoflagellates were first defined by Otto Bütschli in 1885 as the flagellate order Dinoflagellida. Botanists treated them as a division of algae, named Pyrrophyta or Pyrrhophyta ("fire algae"; Greek pyrr(h)os, fire) after the bioluminescent forms, or Dinophyta. At various times, the cryptomonads, ebriids, and ellobiopsids have been included here, but only the last are now considered close relatives. Dinoflagellates have a known ability to transform from noncyst to cyst-forming strategies, which makes recreating their evolutionary history extremely difficult.
Classification
Dinoflagellates are protists which have been classified using both the International Code of Botanical Nomenclature (ICBN, now renamed as ICN) and the International Code of Zoological Nomenclature (ICZN). About half of living dinoflagellate species are autotrophs possessing chloroplasts and half are nonphotosynthesising heterotrophs.
Most (but not all) dinoflagellates have a dinokaryon, described below (see: Lifecycle, below). Dinoflagellates with a dinokaryon are classified under Dinokaryota, while dinoflagellates without a dinokaryon are classified under Syndiniales.
Although classified as eukaryotes, the dinoflagellate nuclei are not characteristically eukaryotic, as some of them lack histones and nucleosomes, and maintain continually condensed chromosomes during mitosis. The dinoflagellate nucleus was termed ‘mesokaryotic’ by Dodge (1966), due to its possession of intermediate characteristics between the coiled DNA areas of prokaryotic bacteria and the well-defined eukaryotic nucleus. This group, however, does contain typically eukaryotic organelles, such as Golgi bodies, mitochondria, and chloroplasts.
Jakob Schiller (1931–1937) provided a description of all the species, both marine and freshwater, known at that time. Later, Alain Sournia (1973, 1978, 1982, 1990, 1993) listed the new taxonomic entries published after Schiller (1931–1937). Sournia (1986) gave descriptions and illustrations of the marine genera of dinoflagellates, excluding information at the species level. The latest index is written by Gómez.
Identification
English-language taxonomic monographs covering large numbers of species are published for the Gulf of Mexico, the Indian Ocean, the British Isles, the Mediterranean and the North Sea.
The main source for identification of freshwater dinoflagellates is the Süsswasser Flora.
Calcofluor-white can be used to stain thecal plates in armoured dinoflagellates.
Morphology
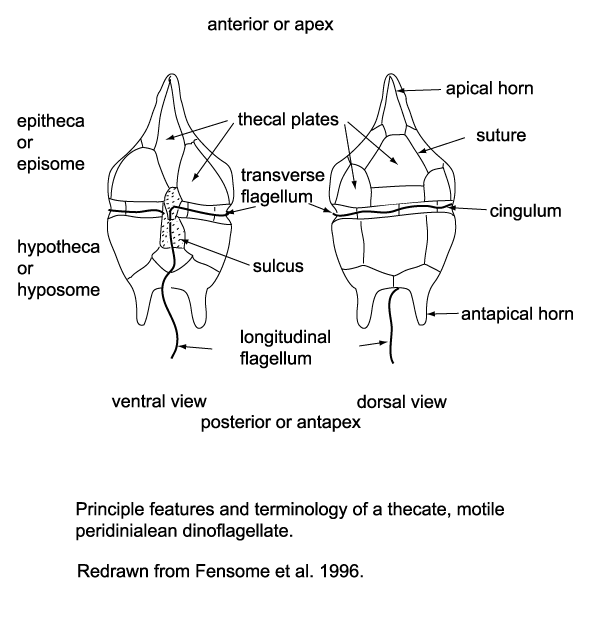
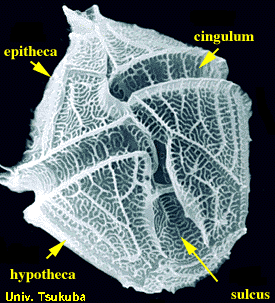
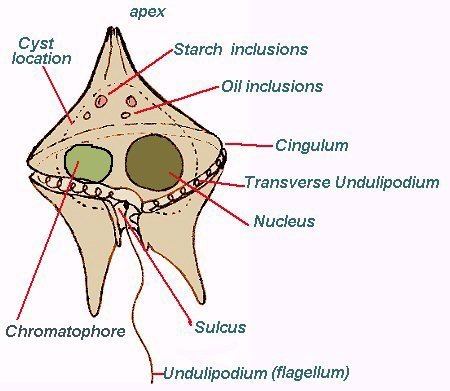
Dinoflagellates are unicellular and possess two dissimilar flagella arising from the ventral cell side (dinokont flagellation). They have a ribbon-like transverse flagellum with multiple waves that beats to the cell's left, and a more conventional one, the longitudinal flagellum, that beats posteriorly. The transverse flagellum is a wavy ribbon in which only the outer edge undulates from base to tip, due to the action of the axoneme which runs along it. The axonemal edge has simple hairs that can be of varying lengths. The flagellar movement produces forward propulsion and also a turning force. The longitudinal flagellum is relatively conventional in appearance, with few or no hairs. It beats with only one or two periods to its wave. The flagella lie in surface grooves: the transverse one in the cingulum and the longitudinal one in the sulcus, although its distal portion projects freely behind the cell. In dinoflagellate species with desmokont flagellation (e.g., Prorocentrum), the two flagella are differentiated as in dinokonts, but they are not associated with grooves.
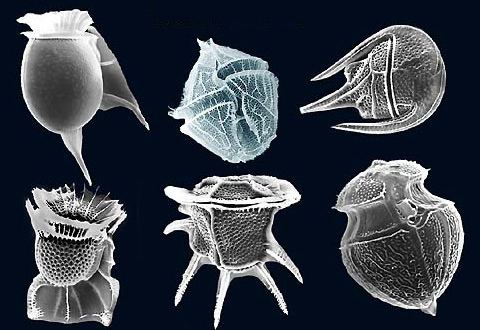
Dinoflagellates have a complex cell covering called an amphiesma or cortex, composed of a series of membrances, flattened vesicles called alveolae (= amphiesmal vesicles) and related structures. In armoured dinoflagellates, these support overlapping cellulose plates to create a sort of armor called the theca, as opposed to athecate dinoflagellates. These occur in various shapes and arrangements, depending on the species and sometimes on the stage of the dinoflagellate. Conventionally, the term tabulation has been used to refer to this arrangement of thecal plates. The plate configuration can be denoted with the plate formula or tabulation formula. Fibrous extrusomes are also found in many forms. Together with various other structural and genetic details, this organization indicates a close relationship between the dinoflagellates, the Apicomplexa, and ciliates, collectively referred to as the alveolates.
Dinoflagellate tabulations can be grouped into six "tabulation types": gymnodinoid, suessoid, gonyaulacoid–peridinioid, nannoceratopsioid, dinophysioid, and prorocentroid.
The chloroplasts in most photosynthetic dinoflagellates are bound by three membranes, suggesting they were probably derived from some ingested algae. Most photosynthetic species contain chlorophylls a and c2, the carotenoid beta-carotene, and a group of xanthophylls that appears to be unique to dinoflagellates, typically peridinin, dinoxanthin, and diadinoxanthin. These pigments give many dinoflagellates their typical golden brown color. However, the dinoflagellates Karenia brevis, Karenia mikimotoi, and Karlodinium micrum have acquired other pigments through endosymbiosis, including fucoxanthin. This suggests their chloroplasts were incorporated by several endosymbiotic events involving already colored or secondarily colorless forms. The discovery of plastids in the Apicomplexa has led some to suggest they were inherited from an ancestor common to the two groups, but none of the more basal lines has them. All the same, the dinoflagellate cell consists of the more common organelles such as rough and smooth endoplasmic reticulum, Golgi apparatus, mitochondria, lipid and starch grains, and food vacuoles. Some have even been found with a light-sensitive organelle, the eyespot or stigma, or a larger nucleus containing a prominent nucleolus. The dinoflagellate Erythropsidium has the smallest known eye.
Some athecate species have an internal skeleton consisting of two star-like siliceous elements that has an unknown function, and can be found as microfossils. Tappan gave a survey of dinoflagellates with internal skeletons. This included the first detailed description of the pentasters in Actiniscus pentasterias, based on scanning electron microscopy. They are placed within the order Gymnodiniales, suborder Actiniscineae.
Endosymbionts
All Zooxanthellae are dinoflagellates and most of them are members within the genus Symbiodinium. The association between Symbiodinium and reef-building corals is widely known. However, endosymbiontic Zooxanthellae inhabit a great number of other invertebrates and protists, for example many sea anemones, jellyfish, nudibranchs, the giant clam Tridacna, and several species of radiolarians and foraminiferans. Many extant dinoflagellates are parasites (here defined as organisms that eat their prey from the inside, i.e. endoparasites, or that remain attached to their prey for longer periods of time, i.e. ectoparasites). They can parasitize animal or protist hosts. Protoodinium, Crepidoodinium, Piscinoodinium, and Blastodinium retain their plastids while feeding on their zooplanktonic or fish hosts. In most parasitic dinoflagellates, the infective stage resembles a typical motile dinoflagellate cell.
Theca structure and formation
The formation of thecal plates has been studied in detail through ultrastructural studies.
Habitats
Dinoflagellates can occur in all aquatic environments: marine, brackish, and fresh water, including in snow or ice. They are also common in benthic environments and sea ice.
Nutritional strategies
Three nutritional strategies are seen in dinoflagellates: phototrophy, mixotrophy, and heterotrophy. Phototrophs can be photoautotrophs or auxotrophs. Mixotrophic dinoflagellates are photosynthetically active, but are also heterotrophic. Facultative mixotrophs, in which autotrophy or heterotrophy is sufficient for nutrition, are classified as amphitrophic. If both forms are required, the organisms are mixotrophic sensu stricto. Some free-living dinoflagellates do not have chloroplasts, but host a phototrophic endosymbiont. A few dinoflagellates may use alien chloroplasts (cleptochloroplasts), obtained from food (kleptoplasty). Some dinoflagellates may feed on other organisms as predators or parasites.
Food inclusions contain bacteria, bluegreen algae, small dinoflagellates, diatoms, ciliates, and other dinoflagellates.
Mechanisms of capture and ingestion in dinoflagellates are quite diverse. Several dinoflagellates, both thecate (e.g. Ceratium hirundinella,; Peridinium globulus,) and nonthecate (e.g. Oxyrrhis marina, Gymnodinium sp. and Kofoidinium spp.), draw prey to the sulcal region of the cell (either via water currents set up by the flagella or via pseudopodial extensions) and ingest the prey through the sulcus. In several Protoperidinium spp., e.g. P. conicum, a large feeding veil — a pseudopod called the pallium — is extruded to capture prey which is subsequently digested extracellularly (= pallium-feeding). Oblea, Zygabikodinium, and Diplopsalis are the only other dinoflagellate genera known to use this particular feeding mechanism ). Katodinium (Gymnodinium) fungiforme, commonly found as a contaminant in algal or ciliate cultures, feeds by attaching to its prey and ingesting prey cytoplasm through an extensible peduncle. The feeding mechanisms of the oceanic dinoflagellates remain unknown, although pseudopodial extensions were observed in Podolampas bipes.
The dinoflagellate nucleus: dinokaryon
Most dinoflagellates have a peculiar form of nucleus, called a dinokaryon, in which the chromosomes are attached to the nuclear membrane. These carry reduced number of histones. In place of histones, dinoflagellate nuclei contain a novel, dominant family of nuclear proteins that appear to be of viral origin, thus are called dinoflagellate/ viral nucleoproteins (DVNPs) which are highly basic, bind DNA with similar affinity to histones, and occur in multiple posttranslationally modified forms. Dinoflagellate nuclei remain condensed throughout interphase rather than just during mitosis, which is closed and involves a uniquely extranuclear mitotic spindle. This sort of nucleus was once considered to be an intermediate between the nucleoid region of prokaryotes and the true nuclei of eukaryotes, so were termed mesokaryotic, but now are considered advanced rather than primitive traits.
Lifecycle
Dinoflagellates have a haplontic lifecycle, with the possible exception of Noctiluca and its relatives. The lifecycle usually involves asexual reproduction by means of binary fission, either through desmoschisis or eleuteroschisis. More complex lifecycles occur, more particularly with parasitic dinoflagellates. Sexual reproduction also occurs, though this mode of reproduction is only known in a small percentage of dinoflagellates. This takes place by fusion of two individuals to form a zygote, which may remain mobile in typical dinoflagellate fashion and is then called a planozygote. This zygote may later form a resting stage or hypnozygote, which is called a dinoflagellate cyst or dinocyst. After (or before) germination of the cyst, the hatchling undergoes meiosis to produce new haploid cells.
Harmful algal blooms
Dinoflagellates sometimes bloom in concentrations of more than a million cells per millilitre. Under such circumstances, they can produce toxins (generally called dinotoxins) in quantities capable of killing fish and accumulating in filter feeders such as shellfish, which in turn may be passed on to people who eat them. This phenomenon is called a red tide, from the color the bloom imparts to the water. Some colorless dinoflagellates may also form toxic blooms, such as Pfiesteria. Some dinoflagellate blooms are not dangerous. Bluish flickers visible in ocean water at night often come from blooms of bioluminescent dinoflagellates, which emit short flashes of light when disturbed.
The same red tide mentioned above is more specifically produced when dinoflagellates are able to reproduce rapidly and copiously on account of the abundant nutrients in the water. Although the resulting red waves are an unusual sight, they contain toxins that not only affect all marine life in the ocean, but the people who consume them, as well. A specific carrier is shellfish. This can introduce both nonfatal and fatal illnesses. One such poison is saxitoxin, a powerful paralytic neurotoxin. Human inputs of phosphate further encourage these red tides, so strong interest exists in learning more about dinoflagellates, from both medical and economic perspectives. The ecology of harmful algal blooms is extensively studied.
Bioluminescence
At night, water can have an appearance of sparkling light due to the bioluminescence of dinoflagellates. More than 18 genera of dinoflagellates are bioluminescent, and the majority of them emit a blue-green light. These species contain scintillons, individual cytoplasmic bodies (about 0.5 µm in diameter) distributed mainly in the cortical region of the cell, outpockets of the main cell vacuole. They contain dinoflagellate luciferase, the main enzyme involved in dinoflagellate bioluminescence, and luciferin, a chlorophyll-derived tetrapyrrole ring that acts as the substrate to the light-producing reaction. The luminescence occurs as a brief (0.1 sec) blue flash (max 476 nm) when stimulated, usually by mechanical disturbance. Therefore, when mechanically stimulated—by boat, swimming, or waves, for example—a blue sparkling light can be seen emanating from the sea surface.
Dinoflagellate bioluminescence is controlled by a circadian clock and only occurs at night. Luminescent and nonluminescent strains can occur in the same species. The number of scintillons is higher during night than during day, and breaks down during the end of the night, at the time of maximal bioluminescence.
The luciferin-luciferase reaction responsible for the bioluminescence is pH sensitive. When the pH drops, luciferase changes its shape, allowing luciferin, more specifically tetrapyrrole, to bind. Dinoflagellates can use bioluminescence as a defense mechanism. They can startle their predators by their flashing light or they can ward off potential predators by an indirect effect such as the "burglar alarm". The bioluminescence attracts attention to the dinoflagellate and its attacker, making the predator more vulnerable to predation from higher trophic levels.
Bioluminescent dinoflagellate ecosystem bays are among the rarest and most fragile, with the most famous ones being the Bioluminescent Bay in La Parguera, Lajas, Puerto Rico; Mosquito Bay in Vieques, Puerto Rico; and Las Cabezas de San Juan Reserva Natural Fajardo, Puerto Rico. Also, a bioluminescent lagoon is near Montego Bay, Jamaica, and bioluminescent harbors surround Castine, Maine.
Lipid and sterol production
Dinoflagellates produce characteristic lipids and sterols. One of these sterols is typical of dinoflagellates and is called dinosterol.
Transport
Dinoflagellate theca can sink rapidly to the seafloor in marine snow.
Genomics
One of their most striking features is the large amount of cellular DNA that dinoflagellates contain. Most eukaryotic algae contain on average about 0.54 Pg DNA/cell, whereas estimates of dinoflagellate DNA content range from 3–250 pg/cell, corresponding to roughly 3000–215 000 Mb (in comparison, the haploid human genome is 3180 Mb and hexaploid Triticum wheat is 16 000 Mb). Polyploidy or polyteny may account for this large cellular DNA content, but studies of DNA reassociation kinetics do not support this hypothesis.
In addition to their disproportionately large genomes, dinoflagellate nuclei are unique in their morphology, regulation, and composition.
The dinoflagellates share an unusual mitochondrial genome organisation with their relatives, the Apicomplexa. Both groups have very reduced mitochondrial genomes (around 6 kilobases in the Apicomplexa). The genes on the dinoflagellate genomes have undergone a number of reorganisations, including massive genome amplification and recombination which have resulted in multiple copies of each gene and gene fragments linked in numerous combinations. Loss of the standard stop codons, trans-splicing of mRNAs for the mRNA of cox3, and extensive RNA editing recoding of most genes has occurred. The reasons for this transformation are unknown.
The DNA of the plastid in the peridinin-containing dinoflagellates is contained in a series of small circles. Each circle contains one or two polypeptide genes. The genes for these polypeptides are chloroplast-specific because their homologs from other photosynthetic eukaryotes are exclusively encoded in the chloroplast genome. Within each circle is a distinguishable 'core' region. Genes are always in the same orientation with respect to this core region.
In terms of DNA barcoding, ITS sequences can be used to identify species, where a genetic distance of p≥0.04 can be used to delimit species.
Evolutionary history
Dinoflagellates are mainly represented as fossils by fossil dinocysts, which have a long geological record with lowest occurrences during the mid-Triassic., whilst geochemical markers suggest a presence to the Early Cambrian.
Some evidence indicates dinosteroids in many Paleozoic and Precambrian rocks might be the product of ancestral dinoflagellates (protodinoflagellates).
Molecular phylogenetics show that dinoflagellates are grouped with ciliates and apicomplexans (=Sporozoa) in a well-supported clade, the alveolates. The closest relatives to dinokaryotic dinoflagellates appear to be apicomplexans, Perkinsus, Parvilucifera, syndinians, and Oxyrrhis. Molecular phylogenies are similar to phylogenies based on morphology.
The earliest stages of dinoflagellate evolution appear to be dominated by parasitic lineages, such as perkinsids and syndinians (e.g. Amoebophrya and Hematodinium).
All dinoflagellates contain red algal plastids or remnant (nonphotosynthetic) organelles of red algal origin. The parasitic dinoflagellate Hematodinium however lacks a plastid entirely.
Dinoflagellate evolution has been summarized into five principal organizational types: prorocentroid, dinophysoid, gonyaulacoid, peridinioid, and gymnodinoid. The transitions of marine species into fresh water have been infrequent events during the diversification of dinoflagellates and in most cases have not occurred recently, possibly as late as the Cretaceous.
Recently, the "living fossil" Dapsilidinium pastielsii was found inhabiting the Indo-Pacific Warm Pool, which served as a refuge for thermophilic dinoflagellates.
