Entrez 7018 | Ensembl ENSG00000091513 | |
 | ||
Aliases TF, PRO1557, PRO2086, TFQTL1, HEL-S-71p, transferrin External IDs OMIM: 190000 MGI: 98821 HomoloGene: 68153 GeneCards: TF | ||
Transferrins are iron-binding blood plasma glycoproteins that control the level of free iron (Fe) in biological fluids. Human transferrin is encoded by the TF gene.
Contents
- Transport mechanism
- Structure
- Tissue distribution
- Immune system
- Role in disease
- Other effects
- Pathology
- Interactions
- Related proteins
- References
Transferrin glycoproteins bind iron tightly, but reversibly. Although iron bound to transferrin is less than 0.1% (4 mg) of total body iron, it forms the most vital iron pool with the highest rate of turnover (25 mg/24 h). Transferrin has a molecular weight of around 80 KDa and contains two specific high-affinity Fe(III) binding sites. The affinity of transferrin for Fe(III) is extremely high (association constant is 1020 M−1 at pH 7.4) but decreases progressively with decreasing pH below neutrality.
When not bound to iron, transferrin is known as "apotransferrin" (see also apoprotein).
Transport mechanism
When a transferrin protein loaded with iron encounters a transferrin receptor on the surface of a cell, e.g., erythroid precursors in the bone marrow, it binds to it and is transported into the cell in a vesicle by receptor-mediated endocytosis. The pH of the vesicle is reduced by hydrogen ion pumps (H+
ATPases) to about 5.5, causing transferrin to release its iron ions. The receptor with its ligand bound transferrin is then transported through the endocytic cycle back to the cell surface, ready for another round of iron uptake. Each transferrin molecule has the ability to carry two iron ions in the ferric form (Fe3+
).
The gene coding for transferrin in humans is located in chromosome band 3q21.
Medical professionals may check serum transferrin level in iron deficiency and in iron overload disorders such as hemochromatosis.
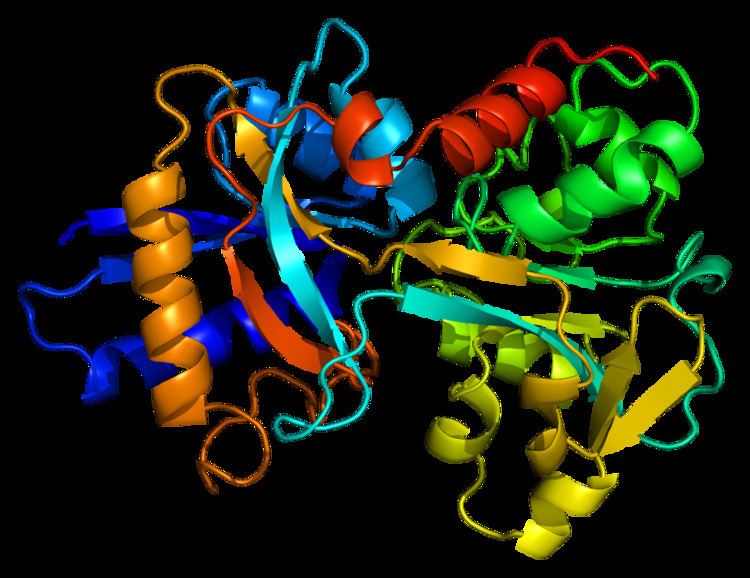
Structure
In humans, transferrin consists of a polypeptide chain containing 679 amino acids and two carbohydrate chains. The protein is composed of alpha helices and beta sheets that form two domains. The N- and C- terminal sequences are represented by globular lobes and between the two lobes is an iron-binding site.
The amino acids which bind the iron ion to the transferrin are identical for both lobes; two tyrosines, one histidine, and one aspartic acid. For the iron ion to bind, an anion is required, preferably carbonate (CO2−
3).
Transferrin also has a transferrin iron-bound receptor; it is a disulfide-linked homodimer. In humans, each monomer consists of 760 amino acids. It enables ligand bonding to the transferrin, as each monomer can bind to one or two atoms of iron. Each monomer consists of three domains: the protease, the helical, and the apical domains. The shape of a transferrin receptor resembles a butterfly based on the intersection of three clearly shaped domains.
Tissue distribution
The liver is the main site of transferrin synthesis but other tissues and organs, including the brain, also produce transferrin. The main role of transferrin is to deliver iron from absorption centers in the duodenum and white blood cell macrophages to all tissues. Transferrin plays a key role in areas where erythropoiesis and active cell division occur. The receptor helps maintain iron homeostasis in the cells by controlling iron concentrations.
Immune system
Transferrin is also associated with the innate immune system. It is found in the mucosa and binds iron, thus creating an environment low in free iron that impedes bacterial survival in a process called iron withholding. The level of transferrin decreases in inflammation.
Role in disease
An increased plasma transferrin level is often seen in patients suffering from iron deficiency anemia, during pregnancy, and with the use of oral contraceptives, reflecting an increase in transferrin protein expression. When plasma transferrin levels rise, there is a reciprocal decrease in percent transferrin iron saturation, and a corresponding increase in total iron binding capacity in iron deficient states A decreased plasma transferrin can occur in iron overload diseases and protein malnutrition. An absence of transferrin results from a rare genetic disorder known as atransferrinemia, a condition characterized by anemia and hemosiderosis in the heart and liver that leads to heart failure and many other complications.
Transferrin and its receptor have been shown to diminish tumour cells when the receptor is used to attract antibodies.
Other effects
Carbohydrate deficient transferrin increases in the blood with heavy ethanol consumption and can be monitored through laboratory testing.
Transferrin is an acute phase protein and is therefore seen to decrease in inflammation, cancers, and certain diseases.
Pathology
Atransferrinemia is associated with a deficiency in transferrin.
In nephrotic syndrome, urinary loss of transferrin, along with other serum proteins such as thyroxine-binding globulin, gammaglobulin, and anti-thrombin III, can manifest as iron-resistant microcytic anemia.
Interactions
Transferrin has been shown to interact with insulin-like growth factor 2 and IGFBP3. Transcriptional regulation of transferrin is upregulated by retinoic acid.
Related proteins
Members of the family include blood serotransferrin (or siderophilin, usually simply called transferrin); lactotransferrin (lactoferrin); milk transferrin; egg white ovotransferrin (conalbumin); and membrane-associated melanotransferrin.
