Symbol Opiods_neuropep InterPro IPR006024 Pfam structures | Pfam PF01160 PROSITE PDOC00964 PDB RCSB PDB; PDBe; PDBj | |
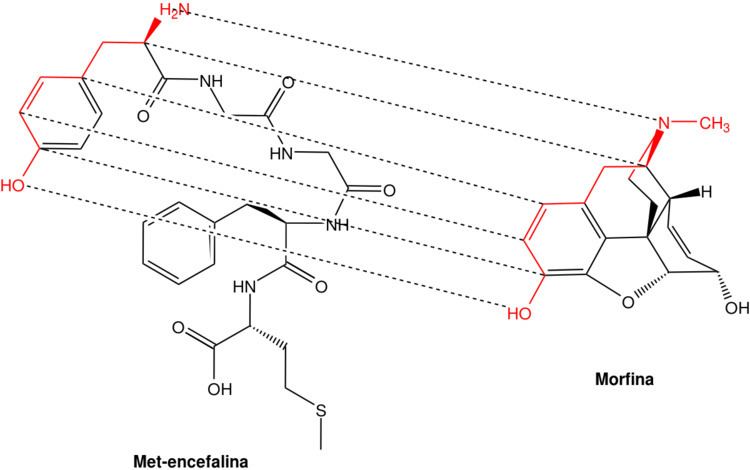 | ||
Opioid peptides are peptides that bind to opioid receptors in the brain; opiates and opioids mimic the effect of these peptides. Such peptides may be produced by the body itself, for example endorphins. The effects of these peptides vary, but they all resemble those of opiates. Brain opioid peptide systems are known to play an important role in motivation, emotion, attachment behaviour, the response to stress and pain, and the control of food intake.
Contents
- Endogenous opioids produced by the body
- Opioid food peptides
- Amphibian opioid peptides
- Synthetic opioid peptides
- References
Opioid-like peptides may also be absorbed from partially digested food (casomorphins, exorphins, and rubiscolins). The opioid food peptides have lengths of typically 4–8 amino acids. The body's own opioids are generally much longer.
Opioid peptides are released by post-translational proteolytic cleavage of precursor proteins. The precursors consist of the following components: a signal sequence that precedes a conserved region of about 50 residues; a variable-length region; and the sequence of the neuropeptides themselves. Sequence analysis reveals that the conserved N-terminal region of the precursors contains 6 cysteines, which are probably involved in disulfide bond formation. It is speculated that this region might be important for neuropeptide processing.
Endogenous opioids produced by the body
The human genome contains several homologous genes that are known to code for endogenous opioid peptides.
