ICD-10-PCS [1] MeSH D016030 MedlinePlus 003005 | ICD-9-CM 55.6 OPS-301 code 5-555 | |
 | ||
Kidney transplantation or renal transplantation is the organ transplant of a kidney into a patient with end-stage renal disease. Kidney transplantation is typically classified as deceased-donor (formerly known as cadaveric) or living-donor transplantation depending on the source of the donor organ. Living-donor renal transplants are further characterized as genetically related (living-related) or non-related (living-unrelated) transplants, depending on whether a biological relationship exists between the donor and recipient. Exchanges and chains are a novel approach to expand the living donor pool. In February 2012, this novel approach to expand the living donor pool resulted in the largest chain in the world, involving 60 participants organized by the National Kidney Registry. In 2014 the record for the largest chain was broken again by a swap involving 70 participants.
Contents
History
One of the earliest mentions about the real possibility of a kidney transplant was by American medical researcher Simon Flexner, who declared in a reading of his paper on “Tendencies in Pathology” in the University of Chicago in 1907 that it would be possible in the then-future for diseased human organs substitution for healthy ones by surgery — including arteries, stomach, kidneys and heart.
In 1933 surgeon Yuriy Voroniy from Kherson in the Soviet Union attempted the first human kidney transplant, using a kidney removed 6 hours earlier from the deceased donor to be reimplanted into the thigh. He measured kidney function using a connection between the kidney and the skin. His first patient died 2 days later as the graft was incompatible with the recipient's blood group and was rejected.
It was not until June 17, 1950, when a successful transplant could be performed on Ruth Tucker, a 44-year-old woman with polycystic kidney disease, at Little Company of Mary Hospital in Evergreen Park, Illinois. Although the donated kidney was rejected ten months later because no immunosuppressive therapy was available at the time—the development of effective antirejection drugs was years away—the intervening time gave Tucker's remaining kidney time to recover and she lived another five years.
The first kidney transplants between living patients were undertaken in 1952 at the Necker hospital in Paris by Jean Hamburger although the kidney failed after 3 weeks of good function and later in 1954 in Boston. The Boston transplantation, performed on December 23, 1954, at Brigham Hospital was performed by Joseph Murray, J. Hartwell Harrison, John P. Merrill and others. The procedure was done between identical twins Ronald and Richard Herrick to eliminate any problems of an immune reaction. For this and later work, Dr. Murray received the Nobel Prize for Medicine in 1990. The recipient, Richard Herrick, died eight years after the transplantation.
In 1955, Charles Rob, William James 'Jim' Dempster (St Marys and Hammersmith, London) carried out the first deceased donor transplant in United Kingdom, which was unsuccessful. In July 1959, 'Fred' Peter Raper (Leeds) performed first successful (8 months) deceased donor transplant in the UK. A year later, in 1960, the first successful living kidney transplant in the UK occurred, when Michael Woodruff performed one between identical twins in Edinburgh. Until the routine use of medications to prevent and treat acute rejection, introduced in 1964, deceased donor transplantation was not performed. The kidney was the easiest organ to transplant: tissue typing was simple, the organ was relatively easy to remove and implant, live donors could be used without difficulty, and in the event of failure, kidney dialysis was available from the 1940s. Tissue typing was essential to the success: early attempts in the 1950s on sufferers from Bright's disease had been very unsuccessful.
The major barrier to organ transplantation between genetically non-identical patients lay in the recipient's immune system, which would treat a transplanted kidney as a "non-self" and immediately or chronically reject it. Thus, having medications to suppress the immune system was essential. However, suppressing an individual's immune system places that individual at greater risk of infection and cancer (particularly skin cancer and lymphoma), in addition to the side effects of the medications.
The basis for most immunosuppressive regimens is prednisolone, a corticosteroid. Prednisolone suppresses the immune system, but its long-term use at high doses causes a multitude of side effects, including glucose intolerance and diabetes, weight gain, osteoporosis, muscle weakness, hypercholesterolemia, and cataract formation. Prednisolone alone is usually inadequate to prevent rejection of a transplanted kidney. Thus other, non-steroid immunosuppressive agents are needed, which also allow lower doses of prednisolone.
Indications
The indication for kidney transplantation is end-stage renal disease (ESRD), regardless of the primary cause. This is defined as a glomerular filtration rate <15ml/min/1.73 sq.m. Common diseases leading to ESRD include malignant hypertension, infections, diabetes mellitus, and focal segmental glomerulosclerosis; genetic causes include polycystic kidney disease, a number of inborn errors of metabolism, and autoimmune conditions such as lupus. Diabetes is the most common known cause of kidney transplantation, accounting for approximately 25% of those in the US. The majority of renal transplant recipients are on dialysis (peritoneal dialysis or hemodialysis) at the time of transplantation. However, individuals with chronic kidney disease who have a living donor available may undergo pre-emptive transplantation before dialysis is needed. If a patient is put on the waiting list for a deceased donor transplant early enough, they may also be transplanted pre-dialysis.
Contraindications and requirements
Contraindications include both cardiac and pulmonary insufficiency, as well as hepatic disease and some cancers. Concurrent tobacco use and morbid obesity are also among the indicators putting a patient at a higher risk for surgical complications.
Kidney transplant requirements vary from program to program and country to country. Many programs place limits on age (e.g. the person must be under a certain age to enter the waiting list) and require that one must be in good health (aside from the kidney disease). Significant cardiovascular disease, incurable terminal infectious diseases and cancer are often transplant exclusion criteria. In addition, candidates are typically screened to determine if they will be compliant with their medications, which is essential for survival of the transplant. People with mental illness and/or significant on-going substance abuse issues may be excluded.
HIV was at one point considered to be a complete contraindication to transplantation. There was fear that immunosuppressing someone with a depleted immune system would result in the progression of the disease. However, some research seem to suggest that immunosuppressive drugs and antiretrovirals may work synergistically to help both HIV viral loads/CD4 cell counts and prevent active rejection.
Compatibility
In general, the donor and recipient should be ABO blood group and crossmatch (HLA antigen) compatible. If a potential living donor is incompatible with their recipient, the donor could be exchange for a compatible kidney. Kidney exchange, also known as "kidney paired donation" or "chains" had recently gained popularity over the past few years.
In an effort to reduce the risk of rejection during incompatible transplantation, ABO-incompatible and densensitization protocols utilizing intravenous immunoglobulin (IVIG) have been developed, with the aim to reduce ABO and HLA antibodies that the recipient may have to the donor.
In the 1980s, experimental protocols were developed for ABO-incompatible transplants using increased immunosuppression and plasmapheresis. Through the 1990s these techniques were improved and an important study of long-term outcomes in Japan was published ([2]). Now, a number of programs around the world are routinely performing ABO-incompatible transplants.
The level of sensitization to donor HLA antigens is determined by performing a panel reactive antibody test on the potential recipient. In the United States, up to 17% of all deceased donor kidney transplants have no HLA mismatch. However, HLA matching is a relatively minor predictor of transplant outcomes. In fact, living non-related donors are now almost as common as living (genetically)-related donors.
Procedure
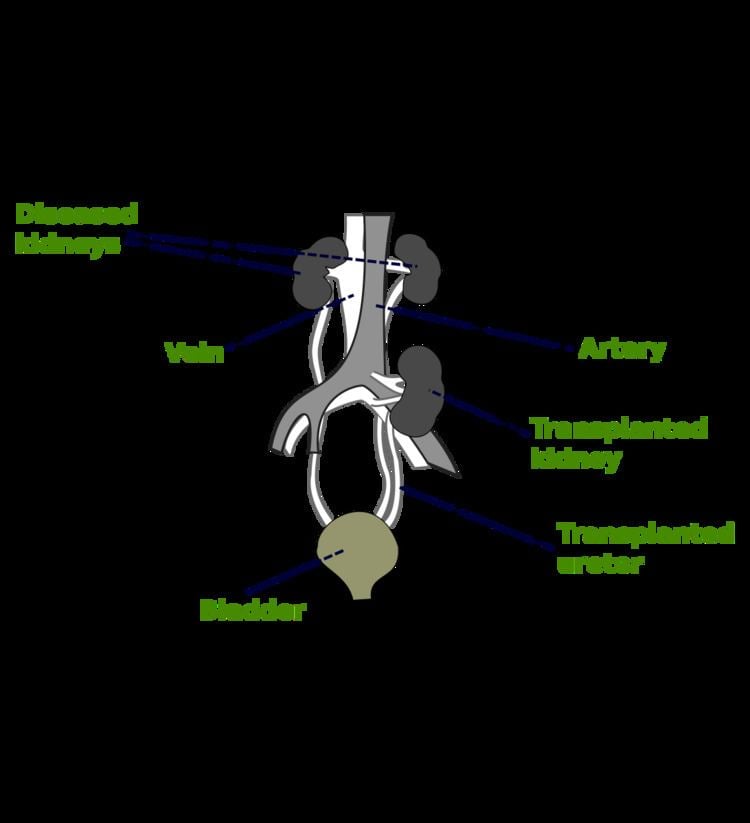
In most cases the barely functioning existing kidneys are not removed, as removal has been shown to increase the rates of surgical morbidity. Therefore, the kidney is usually placed in a location different from the original kidney, often in the iliac fossa, so it is often necessary to use a different blood supply:
There is disagreement in surgical textbooks regarding which side of the recipient’s pelvis to use in receiving the transplant. Campbell's Urology (2002) recommends placing the donor kidney in the recipient’s contralateral side (i.e. a left sided kidney would be transplanted in the recipient's right side) to ensure the renal pelvis and ureter are anterior in the event that future surgeries are required. In an instance where there is doubt over whether there is enough space in the recipient’s pelvis for the donor's kidney, the textbook recommends using the right side because the right side has a wider choice of arteries and veins for reconstruction. Smith's Urology (2004) states that either side of the recipient's pelvis is acceptable; however the right vessels are 'more horizontal' with respect to each other and therefore easier to use in the anastomoses. It is unclear what is meant by the words 'more horizontal'. Glen's Urological Surgery (2004) recommends putting the kidney in the contralateral side in all circumstances. No reason is explicitly put forth; however, one can assume the rationale is similar to that of Campbell, i.e. to ensure that the renal pelvis and ureter are most anterior in the event that future surgical correction becomes necessary.
Kidney-pancreas transplant
Occasionally, the kidney is transplanted together with the pancreas. University of Minnesota surgeons Richard Lillehei and William Kelly perform the first successful simultaneous pancreas-kidney transplant in the world in 1966. This is done in patients with diabetes mellitus type 1, in whom the diabetes is due to destruction of the beta cells of the pancreas and in whom the diabetes has caused renal failure (diabetic nephropathy). This is almost always a deceased donor transplant. Only a few living donor (partial) pancreas transplants have been done. For individuals with diabetes and renal failure, the advantages of earlier transplant from a living donor (if available) are far superior to the risks of continued dialysis until a combined kidney and pancreas are available from a deceased donor. A patient can either receive a living kidney followed by a donor pancreas at a later date (PAK, or pancreas-after-kidney) or a combined kidney-pancreas from a donor (SKP, simultaneous kidney-pancreas).
Transplanting just the islet cells from the pancreas is still in the experimental stage, but shows promise. This involves taking a deceased donor pancreas, breaking it down, and extracting the islet cells that make insulin. The cells are then injected through a catheter into the recipient and they generally lodge in the liver. The recipient still needs to take immunosuppressants to avoid rejection, but no surgery is required. Most people need two or three such injections, and many are not completely insulin-free.
Post operation
The transplant surgery takes about three hours. The donor kidney will be placed in the lower abdomen and its blood vessels connected to arteries and veins in the recipient's body. When this is complete, blood will be allowed to flow through the kidney again. The final step is connecting the ureter from the donor kidney to the bladder. In most cases, the kidney will soon start producing urine.
Depending on its quality, the new kidney usually begins functioning immediately. Living donor kidneys normally require 3–5 days to reach normal functioning levels, while cadaveric donations stretch that interval to 7–15 days. Hospital stay is typically for 4–10 days. If complications arise, additional medications (diuretics) may be administered to help the kidney produce urine.
Immunosuppressant drugs are used to suppress the immune system from rejecting the donor kidney. These medicines must be taken for the rest of the recipient's life. The most common medication regimen today is a mixture of tacrolimus, mycophenolate, and prednisolone. Some recipients may instead take ciclosporin, sirolimus, or azathioprine. The risk of early rejection of the transplanted kidney is increased if corticosteroids are avoided or withdrawn after the transplantation. Ciclosporin, considered a breakthrough immunosuppressive when first discovered in the 1980s, ironically causes nephrotoxicity and can result in iatrogenic damage to the newly transplanted kidney. Tacrolimus, which is a similar drug, also causes nephrotoxicity. Blood levels of both must be monitored closely and if the recipient seems to have declining renal function or proteinuria, a biopsy may be necessary to determine whether this is due to rejection or ciclosporin or tacrolimus intoxication .
Imaging
Post operatively, kidneys are periodically assessed by ultrasound to assess for the imaging and physiologic changes that accompany transplant rejection. Imaging also allows evaluation of supportive structures such as the anastomosed transplant artery, vein, and ureter, to ensure they are stable in appearance.
The major sonographic scale in quantitative ultrasound assessment is with a multipoint assessment of the resistive index (RI), beginning at the main renal artery and vein and ending at the arcuate vessels. It is calculated as follows:
RI = (peak systolic velocity - end diastolic velocity ) / peak systolic velocityThe normal value is ≈ 0.60, with 0.70 being the upper limits of normal.
Postoperative diet
Kidney transplant recipients are discouraged from consuming grapefruit, pomegranate and green tea products. These food products are known to interact with the transplant medications, specifically tacrolimus, cyclosporin and sirolimus; the blood levels of these drugs may be increased, potentially leading to an overdose.
Acute rejection occurs in 10–25% of people after transplant during the first 60 days. Rejection does not necessarily mean loss of the organ, but it may necessitate additional treatment and medication adjustments.
Complications
Problems after a transplant may include: Post operative complication, bleeding, infection, vascular thrombosis and urinary complications
A patient's age and health condition before transplantation affect the risk of complications. Different transplant centers have different success at managing complications and therefore, complication rates are different from center to center.
The average lifetime for a donated kidney is ten to fifteen years. When a transplant fails, a patient may opt for a second transplant, and may have to return to dialysis for some intermediary time.
Infections due to the immunosuppressant drugs used in people with kidney transplants most commonly occur in mucocutaneous areas (41%), the urinary tract (17%) and the respiratory tract (14%). The most common infective agents are bacterial (46%), viral (41%), fungal (13%), and protozoan (1%). Of the viral illnesses, the most common agents are human cytomegalovirus (31.5%), herpes simplex (23.4%), and herpes zoster (23.4%). BK virus is now being increasingly recognised. Infection is the cause of death in about one third of people with renal transplants, and pneumonias account for 50% of the patient deaths from infection.
Prognosis
Kidney transplantation is a life-extending procedure. The typical patient will live 10 to 15 years longer with a kidney transplant than if kept on dialysis. The increase in longevity is greater for younger patients, but even 75-year-old recipients (the oldest group for which there is data) gain an average four more years of life. People generally have more energy, a less restricted diet, and fewer complications with a kidney transplant than if they stay on conventional dialysis.
Some studies seem to suggest that the longer a patient is on dialysis before the transplant, the less time the kidney will last. It is not clear why this occurs, but it underscores the need for rapid referral to a transplant program. Ideally, a kidney transplant should be pre-emptive, i.e., take place before the patient begins dialysis. The reason why kidneys fail over time after transplantation has been elucidated in recent years. Apart from recurrence of the original kidney disease, also rejection (mainly antibody-mediated rejection) and progressive scarring (multifactorial) play a decisive role. Avoiding rejection by strict medication adherence is of utmost importance to avoid failure of the kidney transplant.
At least four professional athletes have made a comeback to their sport after receiving a transplant: New Zealand rugby union player Jonah Lomu, German-Croatian Soccer Player Ivan Klasnić, and NBA basketballers Sean Elliott and Alonzo Mourning.
Statistics
In addition to nationality, transplantation rates differ based on race, sex, and income. A study done with patients beginning long-term dialysis showed that the socio-demographic barriers to renal transplantation are relevant even before patients are on the transplant list. For example, different socio-demographic groups express different interest and complete pre-transplant workup at different rates. Previous efforts to create fair transplantation policies have focused on patients currently on the transplantation waiting list.
In the U.S. health system
Transplant recipients must take immunosuppressive anti-rejection drugs for as long as the transplanted kidney functions. The routine immunosuppressives are tacrolimus (Prograf), mycophenolate (Cellcept), and prednisolone; these drugs cost US$1,500 per month. In 1999 the United States Congress passed a law that restricts Medicare from paying for more than three years for these drugs, unless the patient is otherwise Medicare-eligible. Transplant programs may not transplant a patient unless the patient has a reasonable plan to pay for medication after the Medicare expires; however, patients are almost never turned down for financial reasons alone. Half of end-stage renal disease patients only have Medicare coverage.
In March 2009 a bill was introduced in the U.S. Senate, 565 and in the House, H.R. 1458 that will extend Medicare coverage of the drugs for as long as the patient has a functioning transplant. This means that patients who have lost their jobs and insurance will not also lose their kidney and be forced back on dialysis. Dialysis is currently using up $17 billion yearly of Medicare funds and total care of these patients amounts to over 10% of the entire Medicare budget.
The United Network for Organ Sharing, which oversees the organ transplants in the United States, allows transplant candidates to register at two or more transplant centers, a practice known as 'multiple listing'. The practice has been shown to be effective in mitigating the dramatic geographic disparity in the waiting time for organ transplants, particularly for patients residing in high-demand regions such as Boston. The practice of multiple-listing has also been endorsed by medical practitioners.
