 | ||
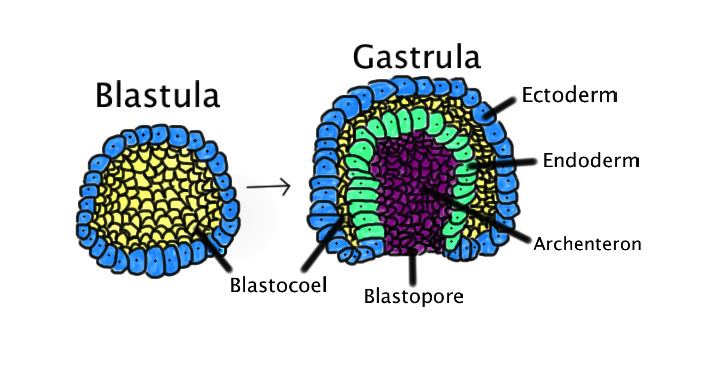
Gastrulation is a phase early in the embryonic development of most animals, during which the single-layered blastula is reorganized into a trilaminar ("three-layered") structure known as the gastrula. These three germ layers are known as the ectoderm, mesoderm, and endoderm.
Contents
- Overview
- Loss of symmetry
- Formation of the primitive streak
- Epithelial to mesenchymal transition and ingression
- Gastrulation in vitro
- References
Gastrulation takes place after cleavage and the formation of the blastula. Gastrulation is followed by organogenesis, when individual organs develop within the newly formed germ layers. Each layer gives rise to specific tissues and organs in the developing embryo. The ectoderm gives rise to epidermis, and to the neural crest and other tissues that will later form the nervous system. The mesoderm is found between the ectoderm and the endoderm and gives rise to somites, which form muscle; the cartilage of the ribs and vertebrae; the dermis, the notochord, blood and blood vessels, bone, and connective tissue. The endoderm gives rise to the epithelium of the digestive system and respiratory system, and organs associated with the digestive system, such as the liver and pancreas. Following gastrulation, cells in the body are either organized into sheets of connected cells (as in epithelia), or as a mesh of isolated cells, such as mesenchyme.
The molecular mechanism and timing of gastrulation is different in different organisms. However, some common features of gastrulation across triploblastic organisms include: (1) A change in the topological structure of the embryo, from a simply connected surface (sphere-like), to a non-simply connected surface (torus-like); (2) the differentiation of cells into one of three types (endodermal, mesodermal, and ectodermal); and (3) the digestive function of a large number of endodermal cells.
Lewis Wolpert, pioneering developmental biologist in the field, has been credited for noting that "It is not birth, marriage, or death, but gastrulation, which is truly the most important time in your life."
The terms "gastrula" and "gastrulation" were coined by Ernst Haeckel, in his 1872 work "Biology of Calcareous Sponges".
Although gastrulation patterns exhibit enormous variation throughout the animal kingdom, they are unified by the five basic types of cell movements that occur during gastrulation: 1) invagination 2) involution 3) ingression 4) delamination 5) epiboly.
Overview
In amniotes (reptiles, birds and mammals), gastrulation involves the creation of the blastopore, an opening into the archenteron. Note that the blastopore is not an opening into the blastocoel, the space within the blastula, but represents a new inpocketing that pushes the existing surfaces of the blastula together. In amniotes, gastrulation occurs in the following sequence: (1) the embryo becomes asymmetric; (2) the primitive streak forms; (3) cells from the epiblast at the primitive streak undergo an epithelial to mesenchymal transition and ingress at the primitive streak to form the germ layers.
The distinction between protostomes and deuterostomes is based on the direction in which the mouth (stoma) develops in relation to the blastopore. Protostome derives from the Greek word protostoma meaning "first mouth"(πρώτος + στόμα) whereas Deuterostome's etymology is "second mouth" from the words second and mouth (δεύτερος + στόμα).
The major distinctions between deuterostomes and protostomes are found in embryonic development:
Loss of symmetry
In preparation for gastrulation, the embryo must become asymmetric along both the proximal-distal axis and the anterior-posterior axis. The proximal-distal axis is formed when the cells of the embryo form the “egg cylinder,” which consists of the extraembryonic tissues, which give rise to structures like the placenta, at the proximal end and the epiblast at the distal end. Many signaling pathways contribute to this reorganization, including BMP, FGF, nodal, and Wnt. Visceral endoderm surrounds the epiblast. The distal visceral endoderm (DVE) migrates to the anterior portion of the embryo, forming the “anterior visceral endoderm” (AVE). This breaks anterior-posterior symmetry and is regulated by nodal signaling.
Formation of the primitive streak
The primitive streak is formed at the beginning of gastrulation and is found at the junction between the extraembryonic tissue and the epiblast on the posterior side of the embryo and the site of ingression. Formation of the primitive streak is reliant upon nodal signaling in the Koller's sickle within the cells contributing to the primitive streak and BMP4 signaling from the extraembryonic tissue. Furthermore, Cer1 and Lefty1 restrict the primitive streak to the appropriate location by antagonizing nodal signaling. The region defined as the primitive streak continues to grow towards the distal tip.
During the early stages of development, the primitive streak is the structure that will establish bilateral symmetry, determine the site of gastrulation and initiate germ layer formation. To form the streak, reptiles, birds and mammals arrange mesenchymal cells along the prospective midline, establishing the first embryonic axis, as well as the place where cells will ingress and migrate during the process of gastrulation and germ layer formation. The primitive streak extends through this midline and creates the antero-posterior body axis, becoming the first symmetry-breaking event in the embryo, and marks the beginning of gastrulation. This process involves the ingression of mesoderm and endoderm progenitors and their migration to their ultimate position, where they will differentiate into the three germ layers. The localization of the cell adhesion and signaling molecule beta-catenin is critical to the proper formation of the organizer region that is responsible for initiating gastrulation.
Epithelial to mesenchymal transition and ingression
In order for the cells to move from the epithelium of the epiblast through the primitive streak to form a new layer, the cells must undergo an epithelial to mesenchymal transition (EMT) to lose their epithelial characteristics, such as cell-cell adhesion. FGF signaling is necessary for proper EMT. FGFR1 is needed for the up regulation of SNAI1, which down regulates E-cadherin, causing a loss of cell adhesion. Following the EMT, the cells ingress through the primitive streak and spread out to form a new layer of cells or join existing layers. FGF8 is implicated in the process of this dispersal from the primitive streak.
Gastrulation in vitro
There have been a number of attempts to understand the processes of Gastrulation using in vitro techniques in parallel and complementary to studies in embryos, usually though the use of 2D and 3D cell (Embryonic organoids) culture techniques using Embryonic stem cells (ESCs) or induced pluripotent stem cells (iPSCs). These are associated with number of clear advantages in using tissue-culture based protocols, some of which include reducing the cost of associated in vivo work (thereby reducing, replacing and refining the use of animals in experiments; the 3Rs), being able to accurately apply agonists/antagonists in spatially and temporally specific manner which may technically difficult to perform during Gastrulation. However, it is important to relate the observations in culture to the processes occurring in the embryo for context.
To illustrate this, the guided differentiation of mouse ESCs has resulted in generating primitive streak-like cells that display many of the characteristics of epiblast cells that traverse through the primitive streak (e.g. transient brachyury up regulation and the cellular changes associated with an epithelial to mesenchymal transition), and human ESCs cultured on micro patterns, treated with BMP4, can generate spatial differentiation pattern similar to the arrangement of the germ layers in the human embryo. Finally, using 3D embryoid body- and organoid-based techniques, small aggregates of mouse ESCs (Embryonic Organoids, or Gastruloids) are able to show a number of processes of early mammalian embryo development such as symmetry-breaking, polarisation of gene expression, gastrulation-like movements, axial elongation and the generation of all three embryonic axes (anteroposterior, dorsoventral and left-right axes).
