ICD-9-CM 425.4 DiseasesDB 3066 | ICD-10 I42.0 OMIM 212110 | |
 | ||
Synonyms Congestive cardiomyopathy, idiopathic cardiomyopathy, primary cardiomyopathy | ||
Dilated cardiomyopathy (DCM) is a condition in which the heart becomes enlarged and cannot pump blood efficiently. The decreased heart function can affect the lungs, liver, and other body systems.
Contents
- Signs and symptoms
- Causes
- Genetics
- Pathophysiology
- Computational models
- Diagnosis
- Treatment
- Surgery
- Reverse remodeling
- Other animals
- References
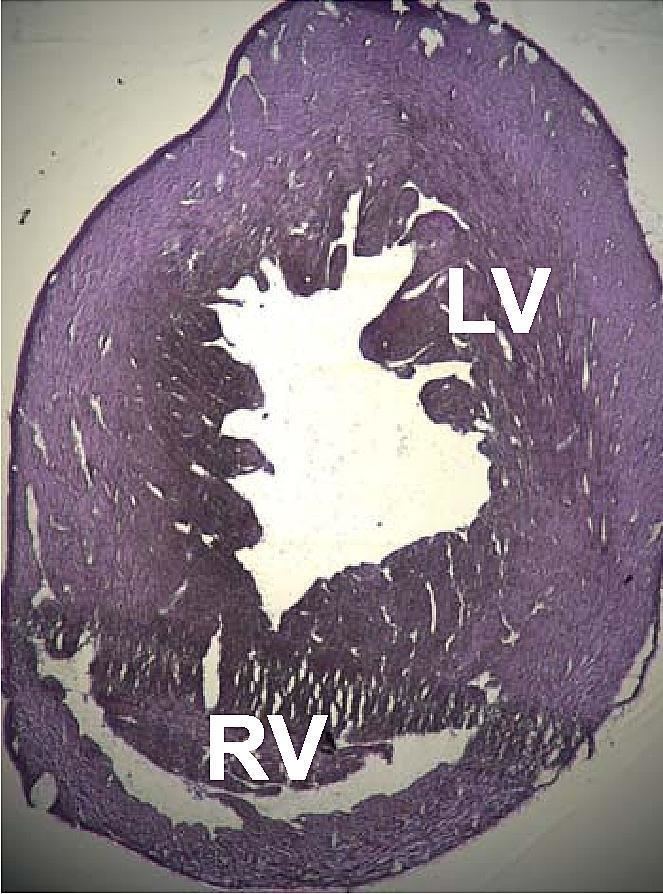
DCM is one of the cardiomyopathies, a group of diseases that affect primarily the heart muscle. Different cardiomyopathies have different causes and affect the heart in different ways. In DCM a portion of the myocardium is dilated, often without any obvious cause. Left or right ventricular systolic pump function of the heart is impaired, leading to progressive heart enlargement via ventricular hypertrophy and ventricular dilation, a process called ventricular remodeling.
Dilated cardiomyopathy is the most common form of non-ischemic cardiomyopathy. It occurs more frequently in men than in women, and is most common between the ages of 20 and 60 years. About one in three cases of congestive heart failure (CHF) is due to dilated cardiomyopathy. Dilated cardiomyopathy also occurs in children.
Signs and symptoms
Dilated cardiomyopathy may not cause symptoms significant enough to impact on quality of life. A minority of people can experience significant symptoms. These might include:
A person suffering from dilated cardiomyopathy may have an enlarged heart, with pulmonary edema and an elevated jugular venous pressure and a low pulse pressure. Signs of mitral and tricuspid regurgitation may be present. A fast heart rate with no change during the respiratory cycle may also be found.
Causes
Although in many cases no cause is apparent, dilated cardiomyopathy is probably the result of damage to the myocardium produced by a variety of toxic, metabolic, or infectious agents. It may be due to fibrous change of the myocardium from a previous myocardial infarction. Or, it may be the late sequelae of acute viral myocarditis, such as with Coxsackie B virus and other enteroviruses possibly mediated through an immunologic mechanism. In cats, taurine deficiency is the most common cause of dilated cardiomyopathy.
Other causes include:
Autoimmune mechanisms are also suggested as a cause for dilated cardiomyopathy.
Recent studies have shown that those subjects with an extremely high occurrence (several thousands a day) of premature ventricular contractions (extrasystole) can develop dilated cardiomyopathy. In these cases, if the extrasystole are reduced or removed (for example, via ablation therapy) the cardiomyopathy usually regresses.
Although the disease is more common in African-Americans than in Caucasians, it may occur in any patient population.
Genetics
About 25–35% of affected individuals have familial forms of the disease, with most mutations affecting genes encoding cytoskeletal proteins, while some affect other proteins involved in contraction. The disease is genetically heterogeneous, but the most common form of its transmission is an autosomal dominant pattern. Autosomal recessive (as found, for example, in Alström syndrome), X-linked (as in Duchenne muscular dystrophy), and mitochondrial inheritance of the disease is also found. Some relatives of those affected by dilated cardiomyopathy have preclinical, asymptomatic heart-muscle changes.
Other cytoskeletal proteins involved in DCM include α-cardiac actin, desmin, and the nuclear lamins A and C. Mitochondrial deletions and mutations presumably cause DCM by altering myocardial ATP generation.
Kayvanpour et al. performed 2016 a meta-analysis with the largest dataset available on genotype-phenotype associations in DCM and mutations in lamin (LMNA), phospholamban (PLN), RNA Binding Motif Protein 20 (RBM20), Cardiac Myosin Binding Protein C (MYBPC3), Myosin Heavy Chain 7 (MYH7), Cardiac Troponin 2 (TNNT2), and Cardiac Troponin I (TNNI3). They also reviewed recent studies investigating genotype-phenotype associations in DCM patients with titin (TTN) mutations. LMNA and PLN mutation carriers showed a high prevalence of cardiac transplantation and ventricular arrhythmia. Dysrhythmias and sudden cardiac death (SCD) was shown to occur even before the manifestation of DCM and heart failure symptoms in LMNA mutation carriers.
Pathophysiology
The progression of heart failure is associated with left ventricular remodeling, which manifests as gradual increases in left ventricular end-diastolic and end-systolic volumes, wall thinning, and a change in chamber geometry to a more spherical, less elongated shape. This process is usually associated with a continuous decline in ejection fraction. The concept of cardiac remodeling was initially developed to describe changes that occur in the days and months following myocardial infarction.
Death is due to either congestive heart failure or ventricular tachy- or bradyarrhythmias.
Computational models
Cardiac dilation is a transversely isotropic, irreversible process resulting from excess strains on the myocardium. A computation model of volumetric, isotropic, and cardiac wall growth predicts the relationship between cardiac strains (e.g. volume overload after myocardial infarction) and dilation using the following governing equations:
where
where
The above model reveals a gradual dilation of the myocardium, especially the ventricular myocardium, to support the blood volume overload in the chambers. Dilation manifests itself in an increase in total cardiac mass and cardiac diameter. Cardiomyocytes reach their maximum length of 150
Diagnosis
Generalized enlargement of the heart is seen upon normal chest X-ray. Pleural effusion may also be noticed, which is due to pulmonary venous hypertension.
The electrocardiogram often shows sinus tachycardia or atrial fibrillation, ventricular arrhythmias, left atrial enlargement, and sometimes intraventricular conduction defects and low voltage. When left bundle-branch block (LBBB) is accompanied by right axis deviation (RAD), the rare combination is considered to be highly suggestive of dilated or congestive cardiomyopathy. Echocardiogram shows left ventricular dilatation with normal or thinned walls and reduced ejection fraction. Cardiac catheterization and coronary angiography are often performed to exclude ischemic heart disease.
Genetic testing can be important, since one study has shown that gene mutations in the TTN gene (which codes for a protein called titin) are responsible for "approximately 25% of familial cases of idiopathic dilated cardiomyopathy and 18% of sporadic cases." The results of the genetic testing can help the doctors and patients understand the underlying cause of the dilated cardiomyopathy. Genetic test results can also help guide decisions on whether a patient's relatives should undergo genetic testing (to see if they have the same genetic mutation) and cardiac testing to screen for early findings of dilated cardiomyopathy.
Cardiac magnetic resonance imaging (cardiac MRI) may also provide helpful diagnostic information in patients with dilated cardiomyopathy.
Treatment
Management and treatment of dilated cardiomyopathy has improved significantly in the last decade. Drug therapy can slow down progression and in some cases even improve the heart condition. Standard therapy may include salt restriction, ACE inhibitors, diuretics, and digitalis. Anticoagulants may also be used. Alcohol should be avoided.
There is some evidence for the benefits of coenzyme Q10 in treating heart failure. Other supplements provided may include L-carnitine, taurine and D-ribose.
Surgery
Artificial pacemakers may be used in patients with intraventricular conduction delay, and implantable cardioverter-defibrillators in those at risk of arrhythmia. These forms of treatment have been shown to improve symptoms and reduce hospitalization.
In patients with advanced disease who are refractory to medical therapy, heart transplantation may be considered.
Reverse remodeling
This refers to reversing the remodeling that has occurred. Therapies that support reverse remodeling have been investigated, and this may suggests a new approach to the prognosis of cardiomyopathies (see ventricular remodeling).
Other animals
Dilated cardiomyopathy is a heritable disease in some dog breeds, including the Boxer, Dobermann, Great Dane, Irish Wolfhound, and St Bernard. Treatment is based on medication, including ACE inhibitors, loop diuretics, and phosphodiesterase inhibitors.
Dilated cardiomyopathy is also a disease affecting some cat breeds, including the Oriental Shorthair, Burmese, Persian, and Abyssinian. As opposed to these hereditary forms, non-hereditary DCM used to be common in the overall cat population before the addition of taurine to commercial cat food.
There is also a high incidence of heritable dilated cardiomyopathy in captive Golden Hamsters (Mesocricetus auratus), due in no small part to their being highly inbred. The incidence is high enough that several strains of Golden Hamster have been developed to serve as animal models in clinical testing for human forms of the disease.
