Formula C10H14O5V Molar mass 265.157 g/mol Boiling point 174 °C | Melting point 258 °C Density 1.5 g/cm³ Appearance blue-green | |
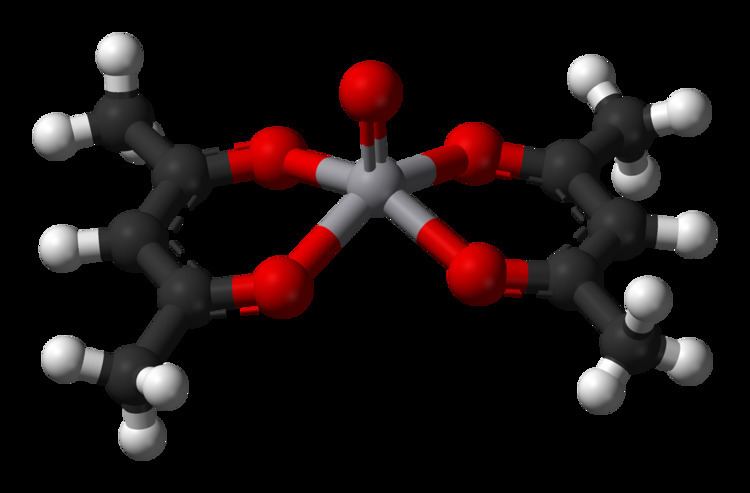 | ||
Vanadyl acetylacetonate is the chemical compound with the formula VO(acac)2, where acac– is the conjugate base of acetylacetone. It is a blue-green solid that dissolves in polar organic solvents. The coordination complex consists of the vanadyl group, VO2+, bound to two acac– ligands via the two oxygen atoms on each. Like other charge-neutral acetylacetonate complexes, it is not soluble in water.
Contents
Synthesis
The complex is generally prepared from vanadium(IV), e.g. vanadyl sulfate:
VOSO4 + 2 Hacac → VO(acac)2 + H2SO4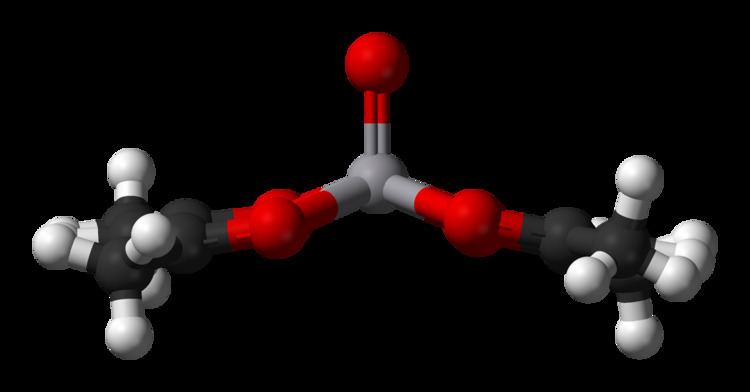
It can also be prepared by a redox reaction starting with vanadium pentoxide. In this reaction, some acetylacetone is oxidized to acetic anhydride.
Structure and properties
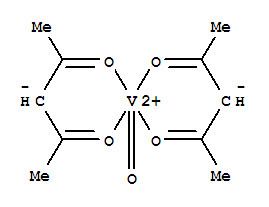
The complex has a square pyramidal structure with a short V=O bond. This d1 compound is paramagnetic. Its optical spectrum exhibits two transitions. It is a weak Lewis acid, forming adducts with pyridine and methylamine.
Applications

It is used in organic chemistry as a catalyst for the epoxidation of allylic alcohols by tert-butyl hydroperoxide (TBHP). The VO(acac)2–TBHP system exclusively epoxidizes geraniol at the allylic alcohol position, leaving the other alkene of geraniol untouched. By comparison, m-CPBA, another epoxidizing agent, reacts with both alkenes, creating the products in a two to one ratio favoring reaction at the alkene away from the hydroxyl group. TBHP oxidizes VO(acac)2 to a vanadium(V) species which coordinates the alcohol of the substrate and the hydroperoxide.
Biomedical aspects

Vanadyl(acac) exhibits insulin mimetic properties, in that in can stimulate the phosphorylation of protein kinase B (PKB/Akt) and glycogen synthase kinase 3 (GSK-3). It has also been shown inhibit tyrosine phosphatase (PTPase), PTPases such as PTP1B, which dephosphorylates insulin receptor beta subunit, thus increasing its phosphorylation, allowing for a prolonged activation of IRS-1, PKB, and GSK-3, allowing them to exert their anti-diabetic properties.
