3DMet B00060 Appearance Colorless gas Formula CH5N Boiling point -6 °C Melting point -93 °C | Abbreviations MMA Related compounds Density 700 kg/m³ Molar mass 31.0571 g/mol | |
 | ||
Related alkanamines | ||
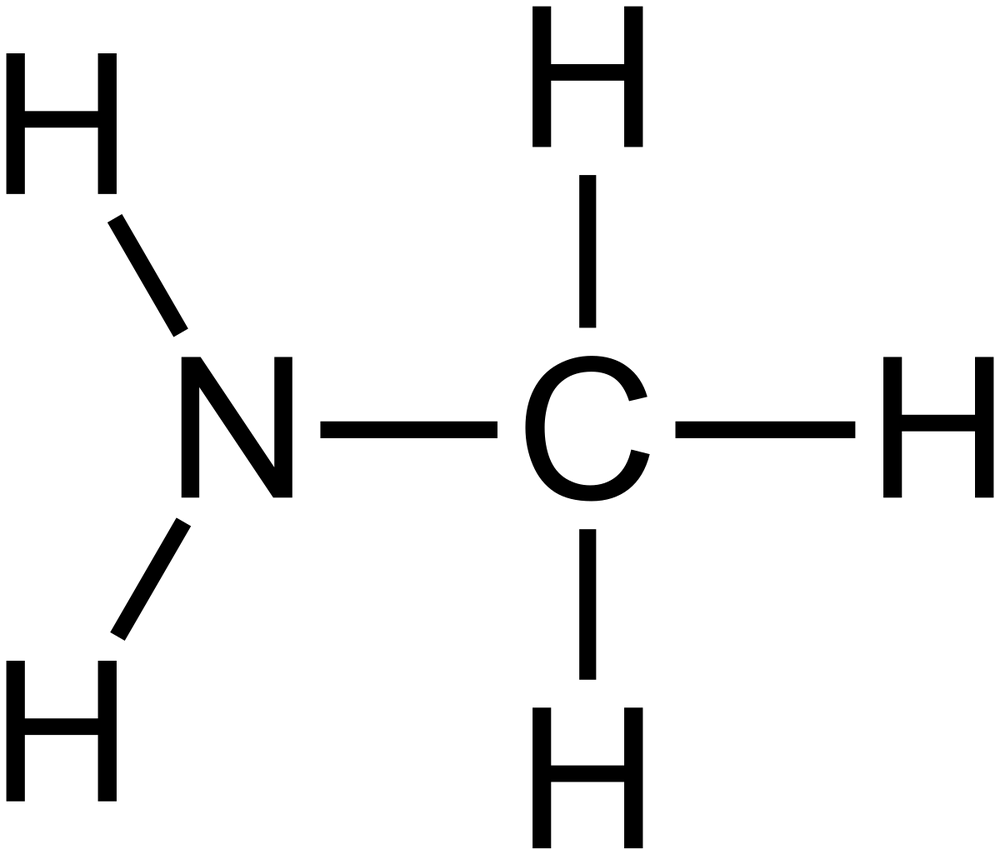
Lewis dot structure of ch3nh2 methylamine
Methylamine is an organic compound with a formula of CH3NH2. This colorless gas is a derivative of ammonia, but with one hydrogen atom being replaced by a methyl group. It is the simplest primary amine. It is sold as a solution in methanol, ethanol, tetrahydrofuran, or water, or as the anhydrous gas in pressurized metal containers. Industrially, methylamine is transported in its anhydrous form in pressurized railcars and tank trailers. It has a strong Odor similar to fish. Methylamine is used as a building block for the synthesis of many other commercially available compounds.
Contents
- Lewis dot structure of ch3nh2 methylamine
- Industrial production
- Laboratory methods
- Reactivity and applications
- Biological chemistry
- Safety
- References

Industrial production
Methylamine is prepared commercially by the reaction of ammonia with methanol in the presence of an aluminosilicate catalyst. dimethylamine and trimethylamine are co-produced; the reaction kinetics and reactant ratios determine the ratio of the three products. The product most favoured by the reaction kinetics is trimethylamine.
CH3OH + NH3 → CH3NH2 + H2OIn this way, an estimated 115,000 tons were produced in 2005.
Laboratory methods

Methylamine was first prepared in 1849 by Charles-Adolphe Wurtz via the hydrolysis of methyl isocyanate and related compounds. An example of this process includes the use of the Hofmann rearrangement, to yield methylamine from acetamide and bromine gas.
In the laboratory methylamine hydrochloride is readily prepared by various other methods. One method entails treating formaldehyde with ammonium chloride.
NH4Cl + H2CO → [CH2=NH2]Cl + H2O[CH2=NH2]Cl + H2CO + H2O → [CH3NH3]Cl + HCO2HThe colorless hydrochloride salt can be converted to an amine by the addition of a strong base, such as sodium hydroxide (NaOH):
[CH3NH3]Cl + NaOH → CH3NH2 + NaCl + H2OAnother method entails reducing nitromethane with zinc and hydrochloric acid.
Reactivity and applications
Methylamine is a good nucleophile as it is highly basic and unhindered, but as an amine it is considered a weak base. Its use in organic chemistry is pervasive. Some reactions involving simple reagents include: with phosgene to methyl isocyanate, with carbon disulfide and sodium hydroxide to the sodium methyldithiocarbamate, with chloroform and base to methyl isocyanide and with ethylene oxide to methylethanolamines. Liquid methylamine has solvent properties analogous to those of liquid ammonia.
Representative commercially significant chemicals produced from methylamine include the pharmaceuticals ephedrine and theophylline, the pesticides carbofuran, carbaryl, and metham sodium, and the solvents N-methylformamide and N-methylpyrrolidone. The preparation of some surfactants and photographic developers require methylamine as a building block.
Biological chemistry
Methylamine arises as a result of putrefaction and is a substrate for methanogenesis.
Additionally, methylamine is produced during PADI4-dependent arginine demethylation.
Safety
The LD50 (mouse, s.c.) is 2.5 g/kg.
The Occupational Safety and Health Administration (OSHA) and National Institute for Occupational Safety and Health (NIOSH) have set occupational exposure limits at 10 ppm or 12 mg/m3 over an eight-hour time-weighted average.
Methylamine is also controlled as a List 1 precursor chemical by the United States Drug Enforcement Administration due to its use in the production of methamphetamine.
