Formula C19H16 Density 1.01 g/cm³ Appearance Colorless solid | Molar mass 244.33 g/mol Boiling point 359 °C | |
Triphenylmethane, or triphenyl methane, is the hydrocarbon with the formula (C6H5)3CH. This colorless solid is soluble in nonpolar organic solvents and not in water. Triphenylmethane is the basic skeleton of many synthetic dyes called triarylmethane dyes, many of them are pH indicators, and some display fluorescence. A trityl group in organic chemistry is a triphenylmethyl group Ph3C, e.g. triphenylmethyl chloride (trityl chloride) and the triphenylmethyl radical (trityl radical).
Contents

How to pronounce triphenylmethane
Preparation

Triphenylmethane was first synthesized in 1872 by the German chemist August Kekulé and his Belgian student Antoine Paul Nicolas Franchimont (1844–1919) by heating diphenylmercury (Hg(C6H5)2, Quecksilberdiphenyl) with benzal chloride (C6H5CHCl2, Benzylenchlorid).
Triphenylmethane can be synthesized by Friedel-Crafts reaction from benzene and chloroform with aluminium chloride catalyst:
3 C6H6 + CHCl3 → Ph3CH + 3 HCl
Alternatively, benzene may react with carbon tetrachloride using the same catalyst to obtain the trityl chloride-aluminium chloride adduct, which is hydrolyzed with dilute acid:
3 C6H6 + CCl4 + AlCl3 → Ph3CCl·AlCl3Ph3CCl·AlCl3 + HCl → Ph3CHSynthesis from benzylidene chloride, prepared from benzaldehyde and phosphorus pentachloride, is used as well.
Acidity
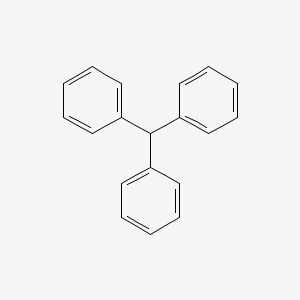
The pKa of the hydrogen on the central carbon is 33. Triphenylmethane is significantly more acidic than most other hydrocarbons because the trityl anion is stabilized by extensive delocalization over three phenyl rings. However, delocalization does not occur simultaneously over all phenyl rings due to steric effects: each phenyl ring forces the other two out of coplanarity to form a three-vaned fan, such that delocalization only occurs if the p-orbital of the anionic carbon is parallel to the p-orbitals of one of the phenyl rings. The trityl anion absorbs strongly in the visible region, making it red. This colour can be used as an indicator when maintaining anhydrous conditions with calcium hydride; the hydride reagent reacts with water to form solid calcium hydroxide, while it is also a strong enough base to generate the trityl anion. If the hydride is used up then the solution will turn colourless. The sodium salt can be prepared also from the chloride:
(C6H5)3CCl + 2 Na → (C6H5)3CNa + NaClBefore the popularization of butyllithium and related strong bases, tritylsodium was often used as a strong, non-nucleophilic base.
Triarylmethane dyes
Examples of triarylmethane dyes are bromocresol green:
And the nitrogen bearing malachite green:

