Formula C4H9NO3 Pubchem 6288 | Molar mass 119.1192 g/mol | |
 | ||
Thermodynamicdata Phase behavioursolid–liquid–gas | ||
Foods rich in threonine foods high in amino acid benefits of wellness
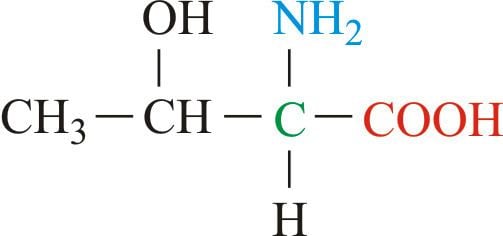
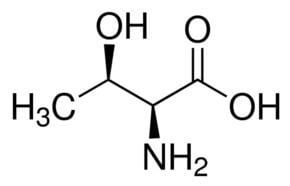
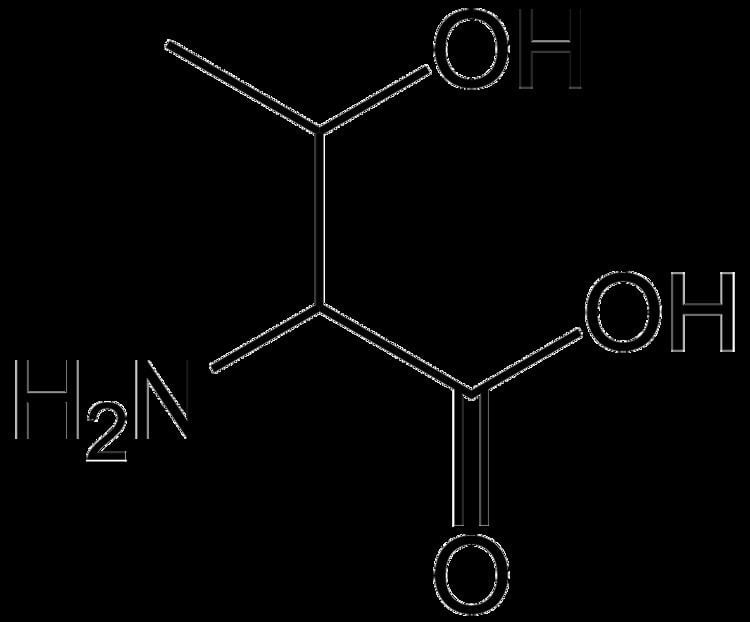
Threonine (abbreviated as Thr or T) encoded by the codons ACU, ACC, ACA, and ACG is an α-amino acid that is used in the biosynthesis of proteins. It contains an α-amino group (which is in the protonated −NH+
3 form under biological conditions), an α-carboxylic acid group (which is in the deprotonated −COO− form under biological conditions), and an alcohol containing side chain, classifying it as a polar, uncharged (at physiological pH) amino acid. It is essential in humans, meaning the body cannot synthesize it, and must be ingested in our diet. Threonine is synthesized from aspartate in bacteria such as E. coli.
Contents
- Foods rich in threonine foods high in amino acid benefits of wellness
- Amino acid digestibility and concentration of de and me in a threonine co product
- Stereoisomerism
- History
- Biosynthesis
- Metabolism
- References

Threonine sidechains are often hydrogen bonded; the commonest small motifs formed are ST turns, ST motifs (often at the beginning of alpha helices) and ST staples (usually at the middle of alpha helices).
Amino acid digestibility and concentration of de and me in a threonine co product
Stereoisomerism
The threonine residue is susceptible to numerous posttranslational modifications. The hydroxyl side-chain can undergo O-linked glycosylation. In addition, threonine residues undergo phosphorylation through the action of a threonine kinase. In its phosphorylated form, it can be referred to as phosphothreonine.
It is a precursor of glycine, and can be used as a prodrug to reliably elevate brain glycine levels.
History
Threonine was discovered as the last of the 20 common proteinogenic amino acids in 1935s by William Cumming Rose, collaborating with Curtis Meyer and William Rose. The amino acid was named threonine because it was similar in structure to threose, a four-carbon monosaccharide or carbohydrate with molecular formula C4H8O4
Threonine is one of two proteinogenic amino acids with two chiral centers, the other being isoleucine. Threonine can exist in four possible stereoisomers with the following configurations: (2S,3R), (2R,3S), (2S,3S) and (2R,3R). However, the name L-threonine is used for one single diastereomer, (2S,3R)-2-amino-3-hydroxybutanoic acid. The second stereoisomer (2S,3S), which is rarely present in nature, is called L-allo-threonine. The two stereoisomers (2R,3S)- and (2R,3R)-2-amino-3-hydroxybutanoic acid are only of minor importance.
Biosynthesis
As an essential amino acid, threonine is not synthesized in humans, hence we must ingest threonine in the form of threonine-containing proteins. In plants and microorganisms, threonine is synthesized from aspartic acid via α-aspartyl-semialdehyde and homoserine. Homoserine undergoes O-phosphorylation; this phosphate ester undergoes hydrolysis concomitant with relocation of the OH group. Enzymes involved in a typical biosynthesis of threonine include:
- aspartokinase
- β-aspartate semialdehyde dehydrogenase
- homoserine dehydrogenase
- homoserine kinase
- threonine synthase.
Metabolism
Threonine is metabolized in two ways:
