Entrez 3728 | Ensembl ENSG00000173801 | |
 | ||
Aliases JUP, ARVD12, CTNNG, DP3, DPIII, PDGB, PKGB, Plakoglobin, junction plakoglobin External IDs MGI: 96650 HomoloGene: 1680 GeneCards: JUP | ||
How to pronounce plakoglobin
Plakoglobin, also known as junction plakoglobin or gamma-catenin, is a protein that in humans is encoded by the JUP gene. Plakoglobin is a member of the catenin protein family and homologous to β-catenin. Plakoglobin is a cytoplasmic component of desmosomes and adherens junctions structures located within intercalated discs of cardiac muscle that function to anchor sarcomeres and join adjacent cells in cardiac muscle. Mutations in plakoglobin are associated with arrhythmogenic right ventricular dysplasia.
Contents
Structure
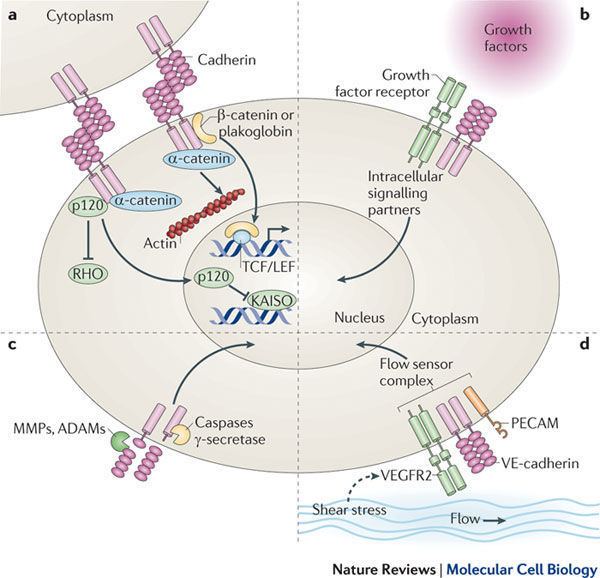
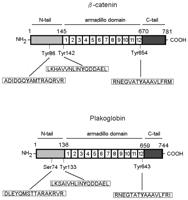
Human plakoglobin is 81.7 kDa in molecular weight and 745 amino acids long. The JUP gene contains 13 exons spanning 17 kb on chromosome 17q21. Plakoglobin is a member of the catenin family, since it contains a distinct repeating amino acid motif called the armadillo repeat. Plakoglobin is highly homologous to β-catenin; both have 12 armadillo repeats as well as N-terminal and C-terminal globular domains of unknown structure. Plakoglobin was originally identified as a component of desmosomes, where it can bind to the cadherin family member desmoglein I. Plakoglobin also associates with classical cadherins such as E-cadherin; in that context, it was called gamma-catenin. Plakoglobin forms distinct complexes with cadherins and desmosomal cadherins.
Function
Plakoglobin is a major cytoplasmic component of both desmosomes and adherens junctions, and is the only known constituent common to submembranous plaques in both of these structures, which are located at the intercalated disc (ICD) of cardiomyocytes. Plakoglobin links cadherins to the actin cytoskeleton. Plakoglobin binds to conserved regions of desmoglein and desmocollin at intracellular catenin-binding sites to assemble desmosomes.
Plakoglobin is essential for normal development of intercalated discs and stability of cardiac muscle. Transgenic mice homozygous for a null mutation of the JUP gene die around embryonic day 12 from substantial defects in adherens junctions and a lack of functional desmosomes in the heart. Further studies showed that cardiac fibers obtained from JUP-null embryonic mice had decreased passive compliance albeit normal attachment of sarcomeres to adherens junctions.
In additional studies, an inducible cardiac-specific plakoglobin knockout mice were generated. Transgenic mice displayed a similar phenotype as arrhythmogenic right ventricular cardiomyopathy patients, with loss of cardiomyocytes, fibrosis and cardiac dysfunction, as well as alterations in desmosome protein content and gap junction remodeling. Hearts also exhibited increases in β-catenin signaling. Further investigations on the role of β-catenin and plakoglobin in the heart generated a double knockout of these two proteins. Mice exhibited cardiomyopathy, fibrosis, conduction abnormalities and sudden cardiac death, presumably via spontaneous lethal ventricular arrhythmias. Mice also showed a decrease in gap junction structures at intercalated discs.
Intracellular plakoglobin expression s controlled by Wnt signaling and ubiquitin-proteasome-dependent degradation. Phosphorylation of N-terminal Serines by a “destruction complex” composed of glycogen synthase kinase 3β (GSK3β) and scaffold proteins adenomatous polyposis coli (APC) and axin targets plakoglobin for degradation.[31–33]. The phosphorylated motif is recognized by β-TrCP, a ubiquitin ligase that targets plakoglobin 26S proteasome-dependent degradation. Plakoglobin is also O-glycosylated near its N-terminal destruction box.
Clinical significance
Mutation of the JUP gene encoding plakoglobin has been implicated as one of the causes of the cardiomyopathy known as arrhythmogenic right ventricular dysplasia (ARVD) or arrhythmogenic right ventricular cardiomyopathy; mutations in JUP specifically causes an autosomal recessive form referred to as Naxos disease. This form of was first identified in a small cluster of families on the Greek island of Naxos. The phenotype of the Naxos disease variant of ARVD is unique in that it involves the hair and skin as well as the right ventricle. Affected individuals have kinky, wooly hair; there is also palmar and plantar erythema at birth that progresses to keratosis as the palms and soles of the feet are used in crawling and walking. These findings co-segregate 100% with the development of ARVD by early adolescence.
It has become clear that ARVD/ARVC is a disease of the cardiac muscle desmosome; advances in molecular genetics have illuminated this notion.
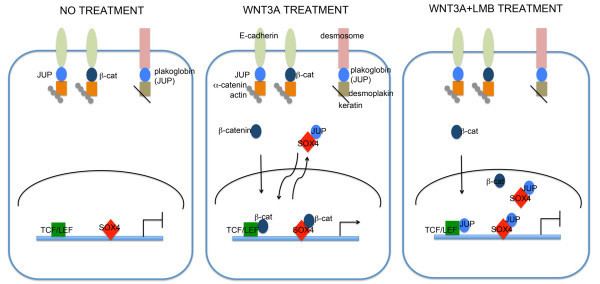
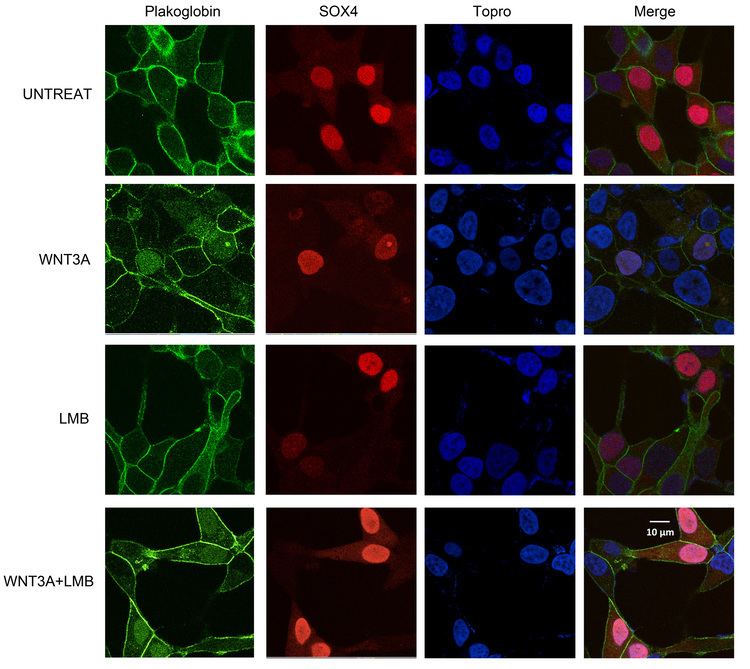
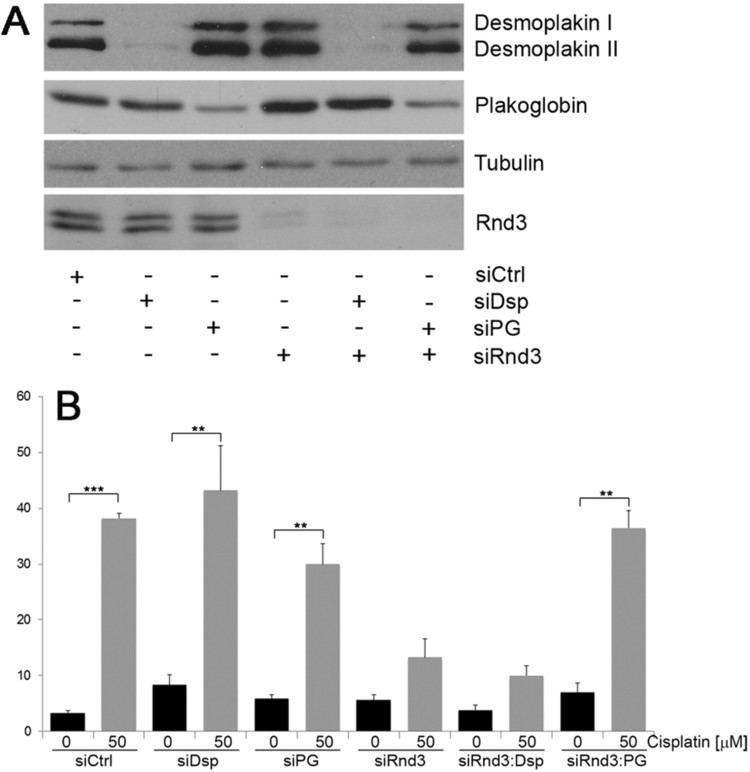
Studies investigating the role of plakoglobin in disease pathology have found that suppression of desmoplakin expression by siRNA led to the nuclear localization of plakoglobin, resulting in a reduction in Wnt signaling via Tcf/Lef1 and ensued pathogenesis of ARVC. Specifically, adipogenic factor expression was induced and cardiac progenitor cells at the epicardium were differentiated to adipocytes.
Non-invasive cardiac screening identified T-wave inversion, abnormalities in right ventricular wall motion, and frequent ventricular extrasystoles as sensitive and specific markers of a JUP mutation. Additional studies have shown that immunohistochemical analysis of cardiac muscle desmosomal proteins is also a sensitive and specific diagnostic text for ARVD/ARVC.
Abnormal distribution of plakoglobin due to mutations in genes encoding for Desmoglein 1 and 3 have also been implicated in Pemphigus vulgaris.
Interactions
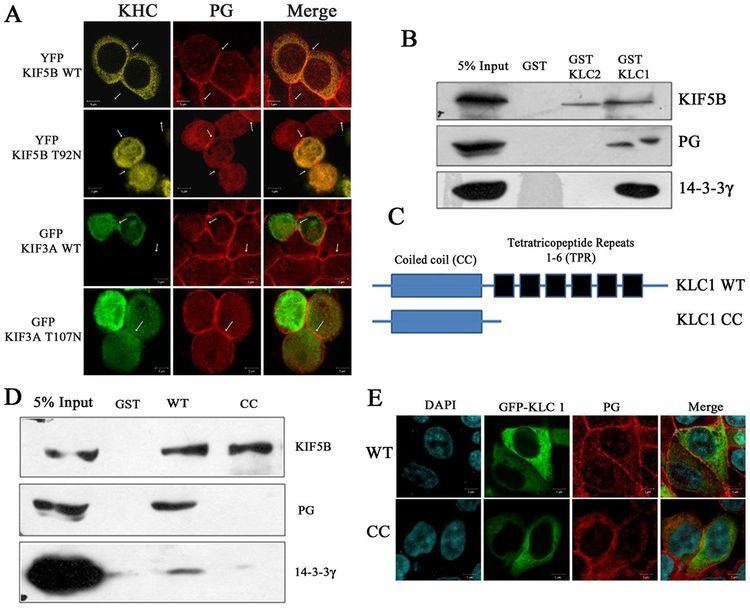
Plakoglobin has been shown to interact with:
