TH H2.00.05.2.02001 | FMA 14068 | |
 | ||
Latin Textus muscularis striatus cardiacus | ||
Cardiac muscle (heart muscle) is an involuntary, striated muscle that is found in the walls and histological foundation of the heart, specifically the myocardium. Cardiac muscle is one of three major types of muscle, the others being skeletal and smooth muscle. These three types of muscle all form in the process of myogenesis. The cells that constitute cardiac muscle, called cardiomyocytes or myocardiocytes, predominantly contain only one nucleus, although populations with two to four nuclei do exist. The myocardium is the muscle tissue of the heart, and forms a thick middle layer between the outer epicardium layer and the inner endocardium layer.
Contents
- Striation
- T tubules
- Intercalated discs
- Physiology
- Regeneration of heart muscle cells
- Clinical significance
- References
Coordinated contractions of cardiac muscle cells in the heart pump blood out of the atria and ventricles to the blood vessels of the left/body/systemic and right/lungs/pulmonary circulatory systems. This complex mechanism illustrates systole of the heart.
Cardiac muscle cells, unlike most other tissues in the body, rely on an available blood and electrical supply to deliver oxygen and nutrients and remove waste products such as carbon dioxide. The coronary arteries help fulfill this function.
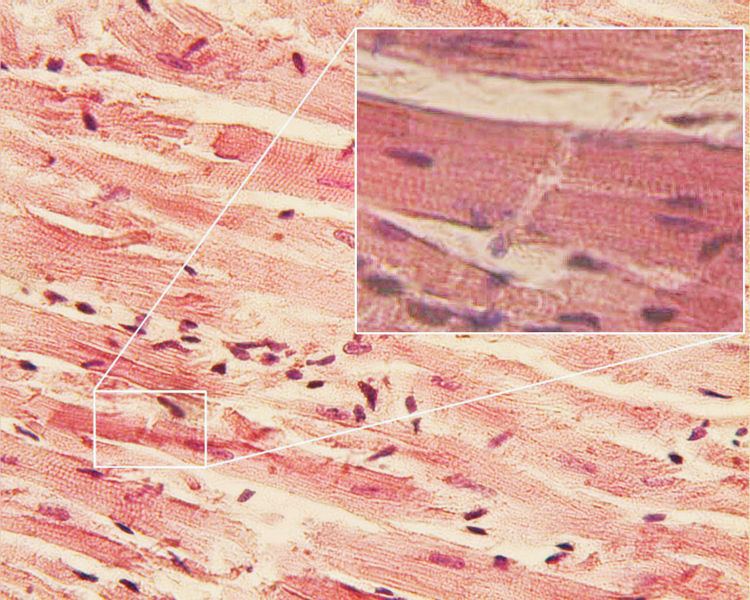
Striation
Cardiac muscle has cross striations formed by rotating segments of thick and thin protein filaments. Like skeletal muscle, the primary structural proteins of cardiac muscle are myosin and actin. The actin filaments are thin, causing the lighter appearance of the I bands in striated muscle, whereas the myosin filament is thicker, lending a darker appearance to the alternating A bands as observed with electron microscopy. However, in contrast to skeletal muscle, cardiac muscle cells are typically branch-like instead of linear.
T-tubules
Another histological difference between cardiac muscle and skeletal muscle is that the T-tubules in the cardiac muscle are bigger and wider and track laterally to the Z-discs. There are fewer T-tubules in comparison with skeletal muscle. The diad is a structure in the cardiac myocyte located at the sarcomere Z-line. It is composed of a single T-tubule paired with a terminal cisterna of the sarcoplasmic reticulum. The diad plays an important role in excitation-contraction coupling by juxtaposing an inlet for the action potential near a source of Ca2+ ions. This way, the wave of depolarization can be coupled to calcium-mediated cardiac muscle contraction via the sliding filament mechanism. Cardiac muscle forms these instead of the triads formed between the sarcoplasmic reticulum in skeletal muscle and T-tubules. T-tubules play critical role in excitation-contraction coupling (ECC). Recently, the action potentials of T-tubules were recorded optically by Guixue Bu et al.
Intercalated discs
The cardiac syncytium is a network of cardiomyocytes connected to each other by intercalated discs that enable the rapid transmission of electrical impulses through the network, enabling the syncytium to act in a coordinated contraction of the myocardium. There is an atrial syncytium and a ventricular syncytium that are connected by cardiac connection fibres. Electrical resistance through intercalated discs is very low, thus allowing free diffusion of ions. The ease of ion movement along cardiac muscle fibers axes is such that action potentials are able to travel from one cardiac muscle cell to the next, facing only slight resistance. Each syncytium obeys the all or none law.
Intercalated discs are complex adhering structures that connect the single cardiomyocytes to an electrochemical syncytium (in contrast to the skeletal muscle, which becomes a multicellular syncytium during mammalian embryonic development). The discs are responsible mainly for force transmission during muscle contraction. Intercalated discs consist of three different types of cell-cell junctions: the actin filament anchoring adherens junctions, the intermediate filament anchoring desmosomes , and gap junctions. They allow action potentials to spread between cardiac cells by permitting the passage of ions between cells, producing depolarization of the heart muscle. However, novel molecular biological and comprehensive studies unequivocally showed that intercalated discs consist for the most part of mixed-type adhering junctions named area composita (pl. areae compositae) representing an amalgamation of typical desmosomal and fascia adhaerens proteins (in contrast to various epithelia). The authors discuss the high importance of these findings for the understanding of inherited cardiomyopathies (such as arrhythmogenic right ventricular cardiomyopathy).
Under light microscopy, intercalated discs appear as thin, typically dark-staining lines dividing adjacent cardiac muscle cells. The intercalated discs run perpendicular to the direction of muscle fibers. Under electron microscopy, an intercalated disc's path appears more complex. At low magnification, this may appear as a convoluted electron dense structure overlying the location of the obscured Z-line. At high magnification, the intercalated disc's path appears even more convoluted, with both longitudinal and transverse areas appearing in longitudinal section.
Physiology
In contrast to skeletal muscle, cardiac muscle requires extracellular calcium ions for contraction to occur. Like skeletal muscle, the initiation and upshoot of the action potential in ventricular cardiomyocytes is derived from the entry of sodium ions across the sarcolemma in a regenerative process. However, an inward flux of extracellular calcium ions through L-type calcium channels sustains the depolarization of cardiac muscle cells for a longer duration. The reason for the calcium dependence is due to the mechanism of calcium-induced calcium release (CICR) from the sarcoplasmic reticulum that must occur during normal excitation-contraction (EC) coupling to cause contraction. Once the intracellular concentration of calcium increases, calcium ions bind to the protein troponin, which allows myosin to bind to actin and contraction to occur.
Regeneration of heart muscle cells
Until recently, it was commonly believed that cardiac muscle cells could not be regenerated. However, a study reported in the April 3, 2009 issue of Science contradicts that belief. Olaf Bergmann and his colleagues at the Karolinska Institute in Stockholm tested samples of heart muscle from people born before 1955 who had very little cardiac muscle around their heart, many showing with disabilities from this abnormality. By using DNA samples from many hearts, the researchers estimated that a 4-year-old renews about 20% of heart muscle cells per year, and about 69 percent of the heart muscle cells of a 50-year-old were generated after he or she was born.
One way that cardiomyocyte regeneration occurs is through the division of pre-existing cardiomyocytes during the normal aging process. The division process of pre-existing cardiomyocytes has also been shown to increase in areas adjacent to sites of myocardial injury. In addition, certain growth factors promote the self-renewal of endogenous cardiomyocytes and cardiac stem cells. For example, insulin-like growth factor 1, hepatocyte growth factor, and high-mobility group protein B1 increase cardiac stem cell migration to the affected area, as well as the proliferation and survival of these cells. Some members of the fibroblast growth factor family also induce cell-cycle re-entry of small cardiomyocytes. Vascular endothelial growth factor also plays an important role in the recruitment of native cardiac cells to an infarct site in addition to its angiogenic effect.
Based on the natural role of stem cells in cardiomyocyte regeneration, researchers and clinicians are increasingly interested in using these cells to induce regeneration of damaged tissue. Various stem cell lineages have been shown to be able to differentiate into cardiomyocytes, including bone marrow stem cells. For example, in one study, researchers transplanted bone marrow cells, which included a population of stem cells, adjacent to an infarct site in a mouse model. Nine days after surgery, the researchers found a new band of regenerating myocardium. However, this regeneration was not observed when the injected population of cells was devoid of stem cells, which strongly suggests that it was the stem cell population that contributed to the myocardium regeneration. Other clinical trials have shown that autologous bone marrow cell transplants delivered via the infarct-related artery decreases the infarct area compared to patients not given the cell therapy.
Clinical significance
Occlusion (blockage) of the coronary arteries by atherosclerosis and/or thrombosis can lead to myocardial infarction (heart attack), where part of the myocardium is injured due to ischemia (not receiving enough oxygen). This occurs because coronary arteries are functional end arteries - i.e. there is almost no overlap in the areas supplied by different arteries (anastomoses) so that if one fails, others cannot adequately perfuse the region, unlike in other tissues.
Certain viruses lead to myocarditis (inflammation of the myocardium). Cardiomyopathies are inherent diseases of the myocardium, many of which are caused by genetic mutations.
