 | ||
In quantum mechanics, the particle in a box model (also known as the infinite potential well or the infinite square well) describes a particle free to move in a small space surrounded by impenetrable barriers. The model is mainly used as a hypothetical example to illustrate the differences between classical and quantum systems. In classical systems, for example, a ball trapped inside a large box, the particle can move at any speed within the box and it is no more likely to be found at one position than another. However, when the well becomes very narrow (on the scale of a few nanometers), quantum effects become important. The particle may only occupy certain positive energy levels. Likewise, it can never have zero energy, meaning that the particle can never "sit still". Additionally, it is more likely to be found at certain positions than at others, depending on its energy level. The particle may never be detected at certain positions, known as spatial nodes.
Contents
- One dimensional solution
- Position wave function
- Momentum wave function
- Position and momentum probability distributions
- Energy levels
- Higher dimensional boxes
- Applications
- Conjugated polyenes
- Quantum well laser
- Quantum dots
- Relativistic Effects
- References
The particle in a box model is one of the very few problems in quantum mechanics which can be solved analytically, without approximations. Due to its simplicity, the model allows insight into quantum effects without the need for complicated mathematics. It serves as a simple illustration of how energy quantization (energy levels), which are found in more complicated quantum systems such as atoms and molecules, come about. It is one of the first quantum mechanics problems taught in undergraduate physics courses, and it is commonly used as an approximation for more complicated quantum systems.
One-dimensional solution
The simplest form of the particle in a box model considers a one-dimensional system. Here, the particle may only move backwards and forwards along a straight line with impenetrable barriers at either end. The walls of a one-dimensional box may be visualised as regions of space with an infinitely large potential energy. Conversely, the interior of the box has a constant, zero potential energy. This means that no forces act upon the particle inside the box and it can move freely in that region. However, infinitely large forces repel the particle if it touches the walls of the box, preventing it from escaping. The potential energy in this model is given as
where L is the length of the box, xc is the location of the center of the box and x is the position of the particle within the box. Simple cases include the centered box (xc = 0 ) and the shifted box (xc = L/2 ).
Position wave function
In quantum mechanics, the wavefunction gives the most fundamental description of the behavior of a particle; the measurable properties of the particle (such as its position, momentum and energy) may all be derived from the wavefunction. The wavefunction
where
Inside the box, no forces act upon the particle, which means that the part of the wavefunction inside the box oscillates through space and time with the same form as a free particle:
where
which is known as the dispersion relation for a free particle. Here one must notice that now, since the particle is not entirely free but under the influence of a potential (the potential V described above), the energy of the particle given above is not the same thing as
The size (or amplitude) of the wavefunction at a given position is related to the probability of finding a particle there by
where
and
where n is a positive integer (1,2,3,4...). For a shifted box (xc = L/2), the solution is particularly simple. The simplest solutions,
Finally, the unknown constant
Thus, A may be any complex number with absolute value √(2/L); these different values of A yield the same physical state, so A = √(2/L) can be selected to simplify.
It is expected that the eigenvalues, i.e., the energy
Momentum wave function
The momentum wavefunction is proportional to the Fourier transform of the position wavefunction. With
where sinc(.) is the sinc function (sinc(x)=sin(x)/x). for the centered box (xc = 0), the solution is particularly simple since the phase factor on the right is unity. Note that in the problematic case of
It can be seen that the momentum spectrum is continuous, and one can conclude that for energy state described by the
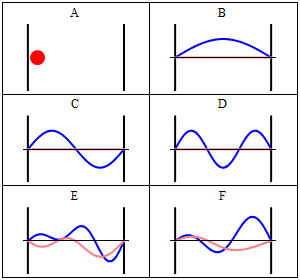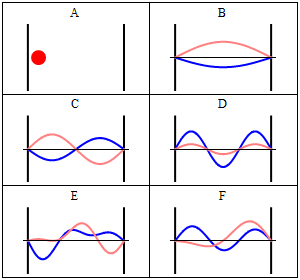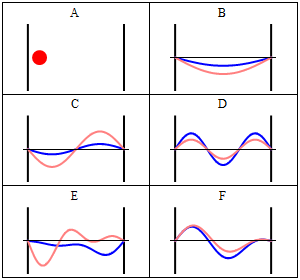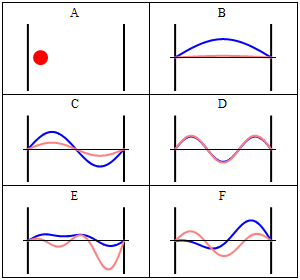
Position and momentum probability distributions
In classical physics, the particle can be detected anywhere in the box with equal probability. In quantum mechanics, however, the probability density for finding a particle at a given position is derived from the wavefunction as
Thus, for any value of n greater than one, there are regions within the box for which
In quantum mechanics, the average, or expectation value of the position of a particle is given by
For the steady state particle in a box, it can be shown that the average position is always
The variance in the position is a measure of the uncertainty in position of the particle:
The probability density for finding a particle with a given momentum is derived from the wavefunction as
where, again,
The uncertainties in position and momentum (
This product increases with increasing n, having a minimum value for n=1. The value of this product for n=1 is about equal to 0.568
Another measure of uncertainty in position is the information entropy of the probability distribution Hx:
where x0 is an arbitrary reference length.
Another measure of uncertainty in momentum is the information entropy of the probability distribution Hp:
where p0 is an arbitrary reference momentum. The integral is difficult to express analytically for general n, but in the limit as n approaches infinity:
where γ is Euler's constant. The quantum mechanical entropic uncertainty principle states that for
For
which satisfies the quantum entropic uncertainty principle.
Energy levels
The energies which correspond with each of the permitted wavenumbers may be written as
The energy levels increase with
The particle, therefore, always has a positive energy. This contrasts with classical systems, where the particle can have zero energy by resting motionlessly. This can be explained in terms of the uncertainty principle, which states that the product of the uncertainties in the position and momentum of a particle is limited by
It can be shown that the uncertainty in the position of the particle is proportional to the width of the box. Thus, the uncertainty in momentum is roughly inversely proportional to the width of the box. The kinetic energy of a particle is given by
Higher-dimensional boxes
If a particle is trapped in a two-dimensional box, it may freely move in the
where the two-dimensional wavevector is given by
For a three dimensional box, the solutions are
where the three-dimensional wavevector is given by:
In general for an n-dimensional box, the solutions are
The 1-dimensional momentum wave functions may likewise be represented by
An interesting feature of the above solutions is that when two or more of the lengths are the same (e.g.
Applications
Because of its mathematical simplicity, the particle in a box model is used to find approximate solutions for more complex physical systems in which a particle is trapped in a narrow region of low electric potential between two high potential barriers. These quantum well systems are particularly important in optoelectronics, and are used in devices such as the quantum well laser, the quantum well infrared photodetector and the quantum-confined Stark effect modulator. It is also used to model a lattice in the Kronig-Penny model and for a finite metal with the free electron approximation.
Conjugated polyenes
Conjugated polyene systems can be modeled using particle in a box. The conjugated system of electrons can be modeled as a one dimensional box with length equal to the total bond distance from one terminus of the polyene to the other. In this case each pair of electrons in each π bond corresponds to one energy level. The energy difference between two energy levels, nf and ni is:
The difference between the ground state energy, n, and the first excited state, n+1, corresponds to the energy required to excite the system. This energy has a specific wavelength, and therefore color of light, related by:
A common example of this phenomenon is in β-carotene. β-carotene (C40H56) is a conjugated polyene with an orange color and a molecular length of approximately 3.8 nm (though its chain length is only approximately 2.4 nm). Due to β-carotene's high level of conjugation, electrons are dispersed throughout the length of the molecule, allowing one to model it as a one-dimensional particle in a box. β-carotene has 11 carbon-carbon double bonds in conjugation; each of those double bonds contains two π-electrons, therefore β-carotene has 22 π-electrons. With two electrons per energy level, β-carotene can be treated as a particle in a box at energy level n=11. Therefore, the minimum energy needed to excite an electron to the next energy level can be calculated, n=12, as follows (recalling that the mass of an electron is 9.109 × 10−31 kg):
Using the previous relation of wavelength to energy, recalling both Planck's constant h and the speed of light c:
This indicates that β-carotene primarily absorbs light in the infrared spectrum, therefore it would appear white to a human eye. However the observed wavelength is 450 nm, indicating that the particle in a box is not a perfect model for this system.
Quantum well laser
The particle in a box model can be applied to quantum well lasers, which are laser diodes consisting of one semiconductor “well” material sandwiched between two other semiconductor layers of different material . Because the layers of this sandwich are very thin (the middle layer is typically about 100 Å thick), quantum confinement effects can be observed. The idea that quantum effects could be harnessed to create better laser diodes originated in the 1970s. The quantum well laser was patented in 1976 by R. Dingle and C. H. Henry
Specifically, the quantum well’s behavior can be represented by the particle in a finite well model. Two boundary conditions must be selected. The first is that the wave function must be continuous. Often, the second boundary condition is chosen to be the derivative of the wave function must be continuous across the boundary, but in the case of the quantum well the masses are different on either side of the boundary. Instead, the second boundary condition is chosen to conserve particle flux as
Due to their small size, quantum dots do not showcase the bulk properties of the specified semi-conductor but rather show quantised energy states. This effect is known as the quantum confinement and has led to numerous applications of quantum dots such as the quantum well laser.
Researchers at Princeton University have recently built a quantum well laser which is no bigger than a grain of rice. The laser is powered by a single electron which passes through two quantum dots; a double quantum dot. The electron moves from a state of higher energy, to a state of lower energy whilst emitting photons in the microwave region. These photons bounce off mirrors to create a beam of light; the laser.
The quantum well laser is heavily based on the interaction between light and electrons. This relationship is a key component in quantum mechanical theories which include the De Broglie Wavelength and Particle in a box. The double quantum dot allows scientists to gain full control over the movement of an electron which consequently results in the production of a laser beam.
Quantum dots
Quantum dots are extremely small semiconductors (on the scale of nanometers). They display quantum confinement in that the electrons cannot escape the “dot”, thus allowing particle-in-a-box approximations to be applied. Their behavior can be described by three-dimensional particle-in-a-box energy quantization equations.
The energy gap of a quantum dot is the energy gap between its valence and conduction bands. This energy gap (E(r)) is equal to the band gap of the bulk material (Egap) plus the energy equation derived from particle-in-a-box, which gives the energy for electrons and holes. This can be seen in the following equation, where m*e and m*h are the effective masses of the electron and hole, r is radius of the dot, and h is Planck's constant:
Hence, the energy gap of the quantum dot is inversely proportional to the square of the “length of the box,” i.e. the radius of the quantum dot.
Manipulation of the band gap allows for the absorption and emission of specific wavelengths of light, as energy is inversely proportional to wavelength. The smaller the quantum dot, the larger the band gap and thus the shorter the wavelength absorbed.
Different semiconducting materials are used to synthesize quantum dots of different sizes and therefore emit different wavelengths of light. Materials that normally emit light in the visible region are often used and their sizes are fine-tuned so that certain colors are emitted. Typical substances used to synthesize quantum dots are cadmium (Cd) and selenium (Se). For example, when the electrons of two nanometer CdSe quantum dots relax after excitation, blue light is emitted. Similarly, red light is emitted in four nanometer CdSe quantum dots.
Quantum dots have a variety of functions including but not limited to fluorescent dyes, transistors, LEDs, solar cells, and medical imaging via optical probes.
One function of quantum dots is their use in lymph node mapping, which is feasible due to their unique ability to emit light in the near infrared (NIR) region. Lymph node mapping allows surgeons to track if and where cancerous cells exist.
Quantum dots are useful for these functions due to their emission of brighter light, excitation by a wide variety of wavelengths, and higher resistance to light than other substances.
Relativistic Effects
The probability density does not go to zero at the nodes if relativistic effects are taken into account via Dirac equation.
