Domain Eukaryota Scientific name Paramecium Order Peniculid | Family Parameciidae Rank Genus | |
Lower classifications Paramecium caudatum, Paramecium aurelia, Paramecium bursaria, Paramecium tetraurelia Similar Paramecium aurelia, Paramecium bursaria, Paramecium caudatum | ||
Paramecium eating pigmented yeast
Paramecium (/ˌpærəˈmiːʃəm, -ˈmiːʃiəm, -ˈmiːsiəm/ parr-ə-MEE-sh(ee-)əm, parr-ə-MEE-see-əm) is a genus of unicellular ciliates, commonly studied as a representative of the ciliate group. Paramecia are widespread in freshwater, brackish, and marine environments and are often very abundant in stagnant basins and ponds. Because some species are readily cultivated and easily induced to conjugate and divide, it has been widely used in classrooms and laboratories to study biological processes. Its usefulness as a model organism has caused one ciliate researcher to characterize it as the "white rat" of the phylum Ciliophora.
Contents
- Paramecium eating pigmented yeast
- Paramecium
- Historical background
- Description
- Movement
- Gathering food
- Symbiosis
- Genome
- Learning
- Reproduction and sexual phenomena
- Aging
- Meiosis and rejuvenation
- List of species
- References
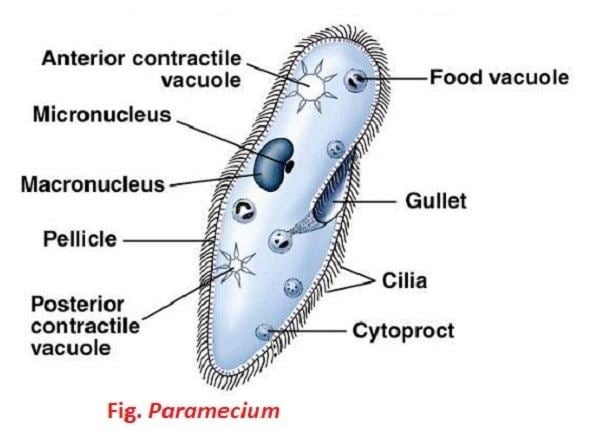
Paramecium
Historical background
Paramecia were among the first ciliates to be seen by microscopists, in the late 17th century. They were probably known to the Dutch pioneer of protozoology, Antonie van Leeuwenhoek, and were clearly described by his contemporary Christiaan Huygens in a letter of 1678. In 1718, the French mathematics teacher and microscopist Louis Joblot published a description and illustration of a microscopic "poisson" (fish), which he discovered in an infusion of oak bark in water. Joblot gave this creature the name "Chausson," or "Slipper," and the phrase "slipper animalcule" remained in use as a colloquial epithet for Paramecium, throughout the 18th and 19th centuries. The name "Paramecium"—constructed from the Greek παραμήκης (paramēkēs, "oblong") -- was coined in 1752 by the English microscopist John Hill, who applied the name generally to "Animalcules which have no visible limbs or tails, and are of an irregularly oblong figure." In 1773, O. F. Müller, the first researcher to place the genus within the Linnaean system of taxonomy, adopted the name Paramecium, but changed the spelling to Paramœcium. C. G. Ehrenberg, in a major study of the infusoria published in 1838, restored Hill's original spelling for the genus name, and most researchers have followed his lead.
Description
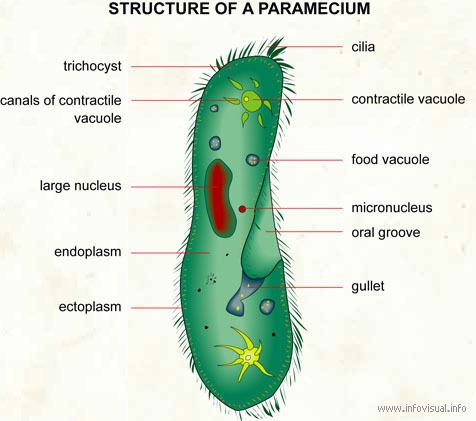
Species of Paramecium range in size from 50 to 330 micrometres (0.0020 to 0.0130 in) in length. Cells are typically ovoid, elongate, foot- or cigar-shaped. The body of the cell is enclosed by a stiff but elastic membrane (pellicle), uniformly covered with simple cilia, hairlike organelles which act like tiny oars to move the organism in one direction. Nearly all species have closely spaced spindle-shaped trichocysts embedded deeply in the cellular envelope (cortex) that surrounds the organism. Typically, an anal pore (cytoproct) is located on the ventral surface, in the posterior half of the cell. In all species, there is a deep oral groove running from the anterior of the cell to its midpoint. This is lined with inconspicuous cilia which beat continuously, drawing food inside the cell. Paramecia live mainly by heterotrophy, feeding on bacteria and other small organisms. A few species are mixotrophs, deriving some nutrients from endosymbiontic algae (chlorella) carried in the cytoplasm of the cell.
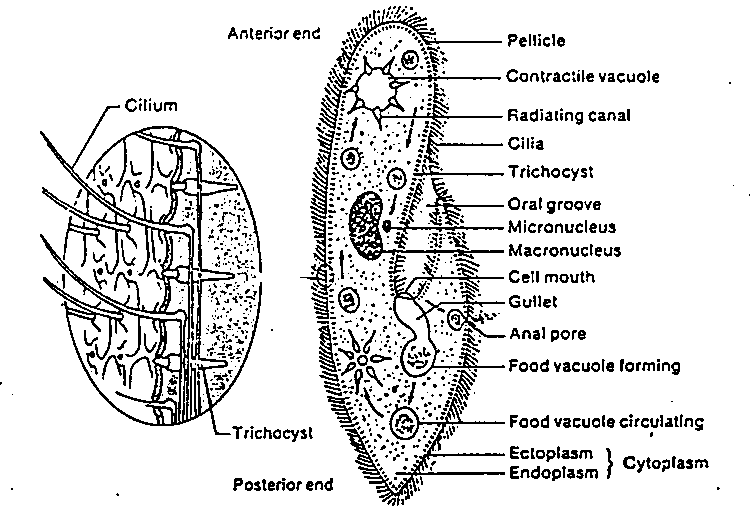
Osmoregulation is carried out by contractile vacuoles, which actively expel water from the cell to compensate for fluid absorbed by osmosis from its surroundings. The number of contractile vacuoles varies from one, to many, depending on species.
Movement
Paramecia propel themselves by whiplash movements of their cilia, which are arranged in tightly spaced rows around the outside of their body. The beat of each cilium has two phases: a fast "effective stroke," during which the cilium is relatively stiff, followed by a slow "recovery stroke," during which the cilium curls loosely to one side and sweeps forward in a counter-clockwise fashion. The densely arrayed cilia move in a coordinated fashion, with waves of activity moving across the "ciliary carpet," creating an effect sometimes likened to that of the wind blowing across a field of grain.
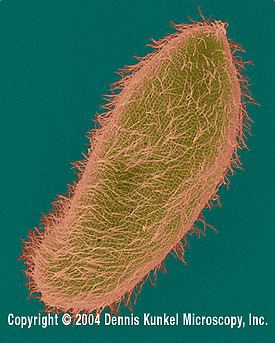
The Paramecium spirals through the water, as it progresses. When it happens to encounter an obstacle, the "effective stroke" of its cilia is reversed and the organism swims backward for a brief time, before resuming its forward progress. This is called the avoidance reaction. If it runs into the solid object again, it will repeat this process, until it can get past the object.
It has been calculated that a Paramecium expends more than half of its energy in propelling itself through the water. Its method of locomotion has been found to be less than 1% efficient. This low percentage is, nevertheless, close to the maximum theoretical efficiency that can be achieved by an organism equipped with cilia as short as those of Paramecium.
Gathering food
Paramecia feed on microorganisms like bacteria, algae, and yeasts. To gather food, the Paramecium uses its cilia to sweep prey organisms, along with some water, through the oral groove, and into the mouth opening. The food passes through the cell mouth into the gullet. When enough food has accumulated at the gullet base, it forms a vacuole in the cytoplasm, which then begins circulating through the cell. As it moves along, enzymes from the cytoplasm enter the vacuole to digest the contents; digested nutrients then pass into the cytoplasm, and the vacuole shrinks. When the vacuole, with its fully digested contents, reaches the anal pore, it ruptures, expelling its waste contents to the environment.
Symbiosis
Some species of Paramecium form mutualistic relationships with other organisms. Paramecium bursaria and Paramecium chlorelligerum harbour endosymbiotic green algae, from which they derive nutrients and a degree of protection from predators such as Didinium nasutum. Numerous bacterial endosymbionts have been identified in species of Paramecium.
Genome
The genome of the species Paramecium tetraurelia has been sequenced, providing evidence for three whole-genome duplications.
In some ciliates, like Stylonychia and Paramecium, only UGA is decoded as a stop codon, while UAG and UAA are reassigned as sense codons.
Learning
The question of whether paramecia exhibit learning has been the object of a great deal of experimentation, yielding equivocal results. However, a study published in 2006 seems to show that Paramecium caudatum may be trained, through the application of a 6.5 volt electric current, to discriminate between brightness levels. This experiment has been cited as a possible instance of cell memory, or epigenetic learning in organisms with no nervous system.
Reproduction and sexual phenomena
Like all ciliates, Paramecia have a dual nuclear apparatus, consisting of a polyploid macronucleus, and one or more diploid micronuclei. The macronucleus controls non-reproductive cell functions, expressing the genes needed for daily functioning. The micronucleus is the generative, or germline nucleus, containing the genetic material that is passed along from one generation to the next.
Paramecia reproduce asexually, by binary fission. During reproduction, the macronucleus splits by a type of amitosis, and the micronuclei undergo mitosis. The cell then divides transversally, and each new cell obtains a copy of the micronucleus and the macronucleus.
Fission may occur spontaneously, in the course of the vegetative cell cycle. Under certain conditions, it may be preceded by self-fertilization (autogamy), or it may follow conjugation, a sexual phenomenon in which Paramecia of compatible mating types fuse temporarily and exchange genetic material. During conjugation, the micronuclei of each conjugant divide by meiosis and the haploid gametes pass from one cell to the other. The gametes of each organism then fuse to form diploid micronuclei. The old macronuclei are destroyed, and new ones are developed from the new micronuclei.
Autogamy or conjugation can be induced by shortage of food at certain points in the Paramecium life cycle.
Aging
In the asexual fission phase of growth, during which cell divisions occur by mitosis rather than meiosis, clonal aging occurs leading to a gradual loss of vitality. In some species, such as the well studied Paramecium tetraurelia, the asexual line of clonally aging paramecia loses vitality and expires after about 200 fissions if the cells fail to undergo autogamy or conjugation. The basis for clonal aging was clarified by transplantation experiments of Aufderheide. When macronuclei of clonally young paramecia were injected into paramecia of standard clonal age, the lifespan (clonal fissions) of the recipient was prolonged. In contrast, transfer of cytoplasm from clonally young paramecia did not prolong the lifespan of the recipient. These experiments indicated that the macronucleus, rather than the cytoplasm, is responsible for clonal aging. Other experiments by Smith-Sonneborn, Holmes and Holmes and Gilley and Blackburn demonstrated that, during clonal aging, DNA damage increases dramatically (also reviewed by Bernstein and Bernstein). Thus, DNA damage in the macronucleus appears to be the cause of aging in P. tetraurelia. In this single-celled protist, aging appears to proceed as it does in multicellular eukaryotes, as described in DNA damage theory of aging.
Meiosis and rejuvenation
When clonally aged P. tetraurelia are stimulated to undergo meiosis in association with either conjugation or automixis, the progeny are rejuvenated, and are able to have many more mitotic binary fission divisions. During either of these processes the micronuclei of the cell(s) undergo meiosis, the old macronucleus disintegrates and a new macronucleus is formed by replication of the micronuclear DNA that had recently undergone meiosis. There is apparently little, if any, DNA damage in the new macronucleus. These findings suggest that clonal aging is due, in large part, to a progressive accumulation of DNA damage (see DNA damage theory of aging); and that rejuvenation is due to the repair of this damage in the micronucleus during meiosis. Meiosis appears to be an adaptation for DNA repair and rejuvenation in these paramecia.
