Symbol Diphtheria_C Pfam clan CL0084 SCOP 1ddt | Pfam PF02763 InterPro IPR022406 SUPERFAMILY 1ddt | |
 | ||
Similar Ricin, Strychnine, Tetrodotoxin | ||
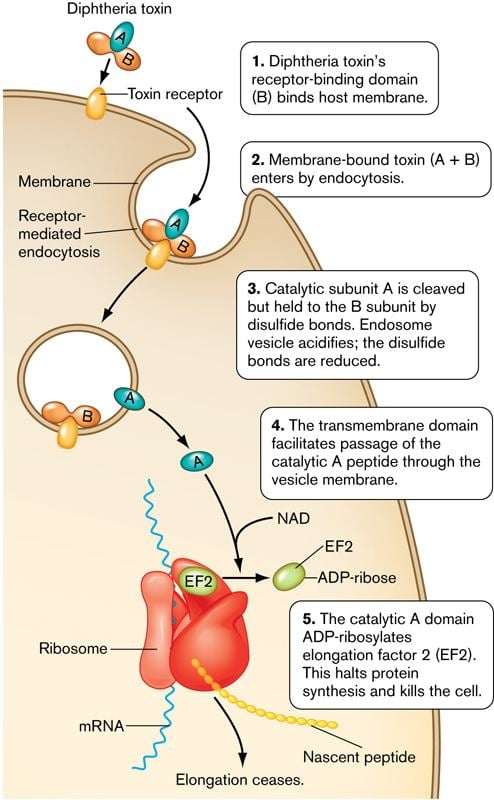
Diphtheria toxin mechanism
Diphtheria toxin is an exotoxin secreted by Corynebacterium diphtheriae, the pathogenic bacterium that causes diphtheria. Unusually, the toxin gene is encoded by a bacteriophage (a virus that infects bacteria). The toxin causes the disease diphtheria in humans by gaining entry into the cell cytoplasm and inhibiting protein synthesis.
Contents
- Diphtheria toxin mechanism
- Characterizing diphtheria toxin antibodies with the proteon system
- Structure
- Mechanism
- Lethal dose and effects
- History
- Clinical use
- References
Characterizing diphtheria toxin antibodies with the proteon system
Structure
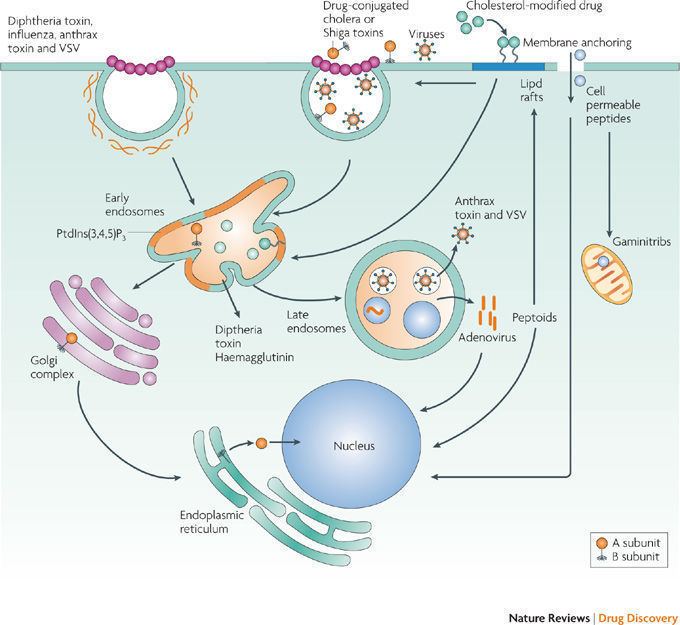
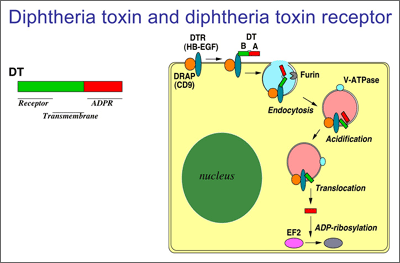
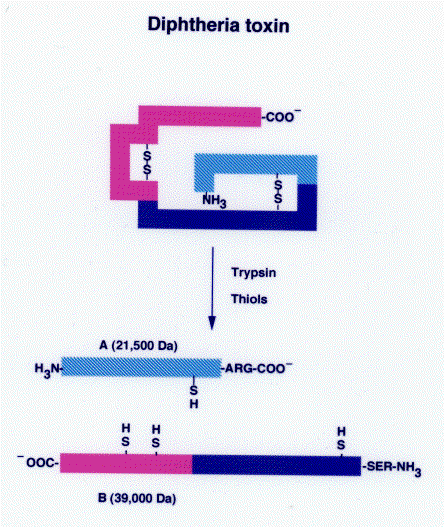
Diphtheria toxin is a single polypeptide chain of 535 amino acids consisting of two subunits linked by disulfide bridges, known as an A-B toxin. Binding to the cell surface of the B subunit (the less stable of the two subunits) allows the A subunit (the more stable part of the protein) to penetrate the host cell.
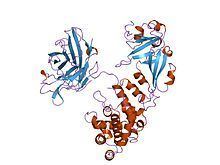
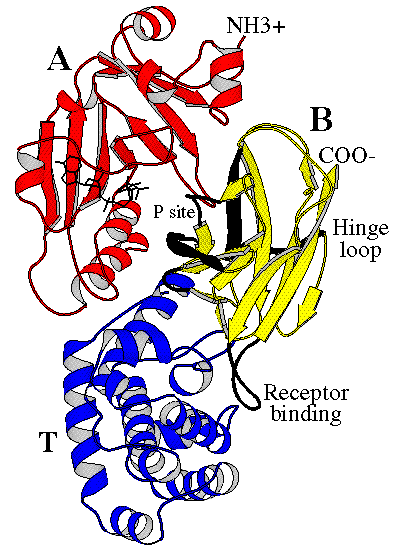
The crystal structure of the diphtheria toxin homodimer has been determined to 2.5 Ångstrom resolution. The structure reveals a Y-shaped molecule consisting of three domains. Fragment A contains the catalytic C domain, and fragment B consists of the T and R domains:
Mechanism
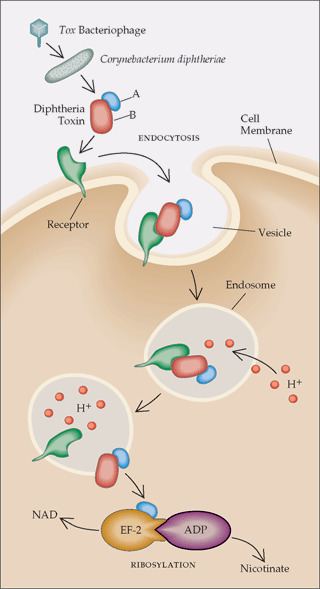
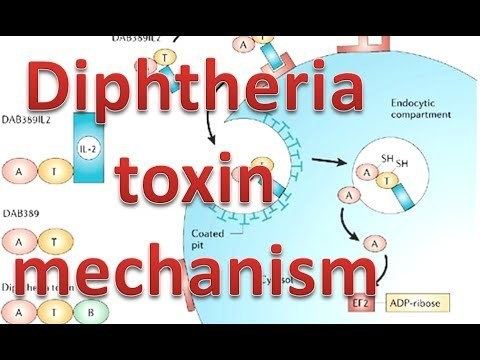
- Processing
- The leader region is cleaved during secretion.
- Proteolytic nicking separates A and B subunits, which remain joined by disulfide bonds until they reach the cytosol.
- The toxin binds to heparin-binding epidermal growth factor precursor (HB-EGF).
- The complex undergoes endocytosis by the host cell.
- Acidification inside the endosome induces translocation of the A subunit into the cytosol.
- Disulfide bonds are broken.
- The B subunit remains in the endosome as a pore.
- A subunit ADP-ribosylates host eEF-2. eEF-2 is required for protein synthesis; when it is inactivated, the host cannot make protein and thus dies.
The diphtheria toxin has the same mechanism of action as the enzyme NAD(+)—Diphthamide ADP-ribosyltransferase (EC 2.4.2.36). It catalyzes the transfer of NAD+ to a diphthamide residue in eEF-2, inactivating this protein. It does so by ADP-ribosylating the unusual amino acid diphthamide. In this way, it acts as a RNA translational inhibitor. The catalysed reaction is as follows:
The exotoxin A of Pseudomonas aeruginosa uses a similar mechanism of action.
Lethal dose and effects
Diphtheria toxin is extraordinarily potent. The lethal dose for humans is about 0.1 μg of toxin per kg of body weight. Death occurs through necrosis of the heart and liver. Diphtheria toxin has also been associated with the development of myocarditis. Myocarditis secondary to diphtheria toxin is considered one of the biggest risks to unimmunized children.
History
Diphtheria toxin was discovered in 1888 by Émile Roux and Alexandre Yersin. In 1890 Emil Adolf von Behring developed an anti-toxin based on blood. In 1951, Freeman found that the toxin gene was not encoded on the bacterial Chromosome, but by a lysogenic phage infecting all toxigenic strains.
Clinical use
The drug denileukin diftitox uses diphtheria toxin as an antineoplastic agent.
Resimmune is an immunotoxin that is in clinical trials in Cutaneous T cell lymphoma patients. It uses diphtheria toxin (truncated by the cell binding domain) coupled to an antibody to CD3ε (UCHT1).
