 | ||
Deoxygenation is a chemical reaction involving the removal of oxygen atoms from a molecule. The term also refers to the removal molecular oxygen (O2) from gases and solvents, a step in air-free technique and gas purifiers. As applied to organic compounds, deoxygenation is a component of fuels production as well a type of reaction employed in organic synthesis, e.g. of pharmaceuticals.
Contents
With replacement by H2
The main examples involving replacement of an oxo group by two hydrogen atoms (A=O → A) is hydrogenolysis. Typical examples use metal catalysts and H2 as the reagent. Conditions are typically more forcing than hydrogenation.
Stoichiometric reactions that effect deoxygenation include the Wolff-Kishner reduction for aryl ketones. The replacement of a hydroxyl group by hydrogen (A-OH → A-H) is the point of the Barton-McCombie deoxygenation and the Markó-Lam deoxygenation.
Other routes
Oxygen groups can also be removed by reductive coupling of ketones, as illustrated by the McMurry reaction.
Epoxides can be deoxygenated using the oxophilic reagent produced by combining tungsten hexachloride and n-butyllithium generates the alkene. This reaction in effect is a de-epoxidation:
P=O bonds
Phosphorus occurs in nature as oxides, so to produce elemental form of the element, deoxygenation is required. The main method involves carbothermic reduction (i.e., carbon is the deoxygenation agent).
4 Ca5(PO4)3F + 18 SiO2 + 30 C → 3 P4 + 30 CO + 18 CaSiO3 + 2 CaF2
Oxophilic main group compounds are useful reagents for certain deoxygenations conducted on laboratory scale. The highly oxophilic reagent hexachlorodisilane (Si2Cl6) stereospecifically deoxygenates phosphine oxides.
S=O bonds
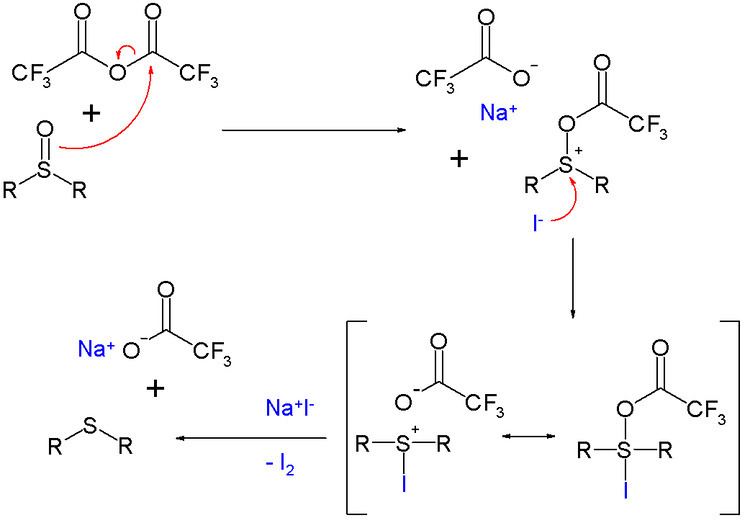
A chemical reagent for the deoxygenation of many sulfur and nitrogen oxo compounds is the combination trifluoroacetic anhydride/sodium iodide. for example in the deoxygenation of the sulfoxide diphenylsulfoxide to the sulfide diphenylsulfide:
The reaction mechanism is based on activation of the sulfoxide by a trifluoroacetyl group and oxidation of iodine. Iodine is formed quantitatively in this reaction and therefore the reagent is used for the analytical detection of many oxo compounds.
