Related compounds Molar mass 146.1427 g/mol Density 935 kg/m³ | Formula C9H6O2 Boiling point 301 °C | |
 | ||
Appearance colorless to white crystals | ||
The coumarin compound
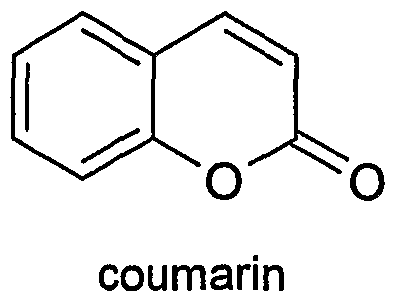
Coumarin (/ˈkuːmərɪn/; 2H-chromen-2-one) is a fragrant organic chemical compound in the benzopyrone chemical class, which is a colorless crystalline substance in its standard state. It is a natural substance found in many plants.
Contents
- The coumarin compound
- Tabac original cologne aroma chemicals coumarin and carcinogens
- History
- Synthesis
- Natural occurrences
- Metabolism
- Metabolism in humans
- Biological function
- Derivatives
- Medical use
- Laser applications
- Toxicity and use in foods beverages cosmetics and tobacco
- Related compounds and derivatives
- Use as pesticides
- References
The name comes from a French term for the tonka bean, coumarou, one of the sources from which coumarin was first isolated as a natural product in 1820. It has a sweet odor, readily recognised as the scent of newly-mown hay, and has been used in perfumes since 1882. Sweet woodruff, meadowsweet, sweet grass and sweet-clover in particular are named for their sweet (i.e., pleasant) smell, which in turn is due to their high coumarin content. When it occurs in high concentrations in forage plants, coumarin is a somewhat bitter-tasting appetite suppressant, and is presumed to be produced by plants as a defense chemical to discourage predation.

Coumarin is used in certain perfumes and fabric conditioners. Coumarin has been used as an aroma enhancer in pipe tobaccos and certain alcoholic drinks, although in general it is banned as a flavorant food additive, due to concerns regarding its hepatotoxicity in animal models.
Coumarin was first synthesized in 1868. It is used in the pharmaceutical industry as a precursor reagent in the synthesis of a number of synthetic anticoagulant pharmaceuticals similar to dicoumarol, the notable ones being warfarin (brand name Coumadin) and some even more potent rodenticides that work by the same anticoagulant mechanism. 4-hydroxycoumarins are a type of vitamin K antagonist. Pharmaceutical (modified) coumarins were all developed from the study of sweet clover disease; see warfarin for this history. However, unmodified coumarin itself, as it occurs in plants, has no effect on the vitamin K coagulation system, or on the action of warfarin-type drugs.
Coumarin has clinical medical value by itself, as an edema modifier. Coumarin and other benzopyrones, such as 5,6-benzopyrone, 1,2-benzopyrone, diosmin, and others, are known to stimulate macrophages to degrade extracellular albumin, allowing faster resorption of edematous fluids. Other biological activities that may lead to other medical uses have been suggested, with varying degrees of evidence.
Coumarin is also used as a gain medium in some dye lasers, and as a sensitizer in older photovoltaic technologies.
Tabac original cologne aroma chemicals coumarin and carcinogens
History
The word tonka for tonka beans is taken from the Galibi (Carib) tongue spoken by natives of French Guiana (one source for the plant); it also appears in Old Tupi, another language of the same region, as the name of the tree. The old genus name, Coumarouna, was formed from another Tupi name for tree, kumarú. The French word for the tonka bean, coumarou, is from this name.
Coumarin, named for coumarou was first isolated from Tonka beans and sweet clover in 1820 by A. Vogel of Munich, who initially mistook it for benzoic acid. Also in 1820, Nicholas Jean Baptiste Gaston Guibourt (1790–1867) of France independently isolated coumarin, but he realized that it was not benzoic acid. In a subsequent essay he presented to the pharmacy section of the Académie Royale de Médecine, Guibourt named the new substance "coumarine". In 1835, the French pharmacist A. Guillemette proved that Vogel and Guibourt had isolated the same substance. Coumarin was first synthesized in 1868 by the English chemist William Henry Perkin.
Synthesis
Coumarin can be prepared by a number of name reactions with the Perkin reaction between salicylaldehyde and acetic anhydride being a popular example. The Pechmann condensation provides another route to coumarin and its derivatives; as does the Kostanecki acylation which can also be used to produce chromones.
Natural occurrences
Coumarin is found naturally in many plants, notably in high concentration in the tonka bean (Dipteryx odorata), vanilla grass (Anthoxanthum odoratum), sweet woodruff (Galium odoratum), mullein (Verbascum spp.), sweet grass (Hierochloe odorata), cassia cinnamon (Cinnamomum cassia) not to be confused with true cinnamon ("Ceylon cinnamon", Cinnamomum zeylanicum which contains little coumarin), sweet-clover (Melilotus sp.), deertongue (Dichanthelium clandestinum), and the leaves of many cherry blossom tree varieties (of the Prunus genus). Coumarin is also found in extracts of Justicia pectoralis.
Related compounds are found in some but not all specimens of licorice.
Metabolism
The biosynthesis of coumarin in plants is via hydroxylation, glycolysis, and cyclization of cinnamic acid.
Metabolism in humans
The enzyme encoded by the gene UGT1A8 has glucuronidase activity with many substrates including coumarins.
Biological function
Coumarin has appetite-suppressing properties, which may discourage animals from eating plants which contain it. Though the compound has a pleasant sweet odor, it has a bitter taste, and animals tend to avoid it.
Derivatives
Coumarin and its derivatives are all considered phenylpropanoids.
Some naturally occurring coumarin derivatives include umbelliferone (7-hydroxycoumarin), aesculetin (6,7-dihydroxycoumarin), herniarin (7-methoxycoumarin), psoralen and imperatorin.
4-Phenylcoumarin is the backbone of the neoflavones, a type of neoflavonoids.
Medical use
Coumarins have shown some evidence of biological activity and have limited approval for few medical uses as pharmaceuticals, such as in the treatment of lymphedema and their ability to increase plasma antithrombin levels. Both coumarin and indandione derivatives produce a uricosuric effect, by interfering with the renal tubular reabsorption of urate.
Laser applications
Coumarin dyes are extensively used as gain media in blue-green tunable organic dye lasers. Among the various coumarin laser dyes are coumarins 480, 490, 504, 521, 504T, and 521T. Coumarin tetramethyl laser dyes offer wide tunability and high laser gain, and they are also used as active medium in coherent OLED emitters.
Toxicity and use in foods, beverages, cosmetics, and tobacco
Coumarin is moderately toxic to the liver and kidneys, with a median lethal dose (LD50) of 275 mg/kg, a low toxicity compared to related compounds. Though it is only somewhat dangerous to humans, coumarin is hepatotoxic in rats, but less so in mice. Rodents metabolize it mostly to 3,4-coumarin epoxide, a toxic, unstable compound that on further differential metabolism may cause liver cancer in rats and lung tumors in mice. Humans metabolize it mainly to 7-hydroxycoumarin, a compound of lower toxicity. The German Federal Institute for Risk Assessment has established a tolerable daily intake (TDI) of 0.1 mg coumarin per kg body weight, but also advises that higher intake for a short time is not dangerous. The Occupational Safety and Health Administration (OSHA) of the United States does not classify coumarin as a carcinogen for humans.
European health agencies have warned against consuming high amounts of cassia bark, one of the four main species of cinnamon, because of its coumarin content. According to the German Federal Institute for Risk Assessment (BFR), 1 kg of (cassia) cinnamon powder contains about 2.1 to 4.4 g of coumarin. Powdered cassia cinnamon weighs 0.56 g/cm3, so a kilogram of cassia cinnamon powder equals 362.29 teaspoons. One teaspoon of cassia cinnamon powder therefore contains 5.8 to 12.1 mg of coumarin, which may be above the tolerable daily intake value for smaller individuals. However, the BFR only cautions against high daily intake of foods containing coumarin. Its report specifically states that Ceylon cinnamon (Cinnamomum verum) contains "hardly any" coumarin.
The European Regulation (EC) No 1334/2008 describes the following maximum limits for coumarin: 50 mg/kg in traditional and/or seasonal bakery ware containing a reference to cinnamon in the labeling, 20 mg/kg in breakfast cereals including muesli, 15 mg/kg in fine bakery ware, with the exception of traditional and/or seasonal bakery ware containing a reference to cinnamon in the labeling, and 5 mg/kg in desserts. An investigation from the Danish Veterinary and Food Administration in 2013 shows that bakery goods characterized as fine bakery ware exceeds the European limit (15 mg/kg) in almost 50% of the cases. The paper also mentions tea as an additional important contributor to the overall coumarin intake, especially for children with a sweet habit.
Coumarin is found naturally in many edible plants such as strawberries, black currants, apricots, and cherries.
Coumarin is often found in artificial vanilla substitutes, despite having been banned as a food additive in numerous countries since the mid-20th century. Coumarin was banned as a food additive in the United States in 1954, largely because of the hepatotoxicity results in rodents. Coumarin is currently listed by the Food and Drug Administration (FDA) of the United States among "Substances Generally Prohibited From Direct Addition or Use as Human Food", according to 21 CFR 189.130, but some natural additives containing coumarin, such as the flavorant sweet woodruff are allowed "in alcoholic beverages only" (21 CFR 172.510). In Europe, popular examples of such beverages are Maiwein (white wine with woodruff) and Żubrówka (vodka flavoured with bison grass).
Coumarin is subject to restrictions on its use in perfumery, as some people may become sensitized to it, however the evidence that coumarin can cause an allergic reaction in humans is disputed.
Minor neurological dysfunction was found in children exposed to coumarin during pregnancy. 306 children were tested at ages 7–15 years to determine subtle neurological effects from coumarin exposure. Results showed a dose response relationship between coumarin exposure and minor neurological dysfunction. Overall, a 1.9 (90%) increase in minor neurological dysfunction was observed for children exposed to coumarin. In conclusion, researchers stated, "The results suggest that coumarins have an influence on the development of the brain which can lead to mild neurologic dysfunctions in children of school age."
Coumarin is still used as a legal flavorant in the tobacco industry, particularly for sweet pipe tobacco. Its presence in cigarette tobacco led former Brown & Williamson executive Jeffrey Wigand to contact CBS's news show 60 Minutes in 1995, charging a “form of rat poison” was in the tobacco. He held that from a chemist’s point of view, coumarin is an “immediate precursor” to the rodenticide coumadin. Dr. Wigand later stated that coumarin itself is dangerous, pointing out that the FDA had banned its addition to human food in 1954. Under his later testimony he would repeatedly classify coumarin as a "lung-specific carcinogen". In Germany, coumarin is banned as additive in tobacco.
Alcoholic beverages sold in the European Union are limited to a maximum of 10 mg/l coumarin by law. Cinnamon flavor is generally cassia bark steam-distilled to concentrate the cinnamaldehyde, for example, to about 93%. Clear cinnamon-flavored alcoholic beverages generally test negative for coumarin, but if whole cassia bark is used to make mulled wine, then coumarin shows up in significant levels.
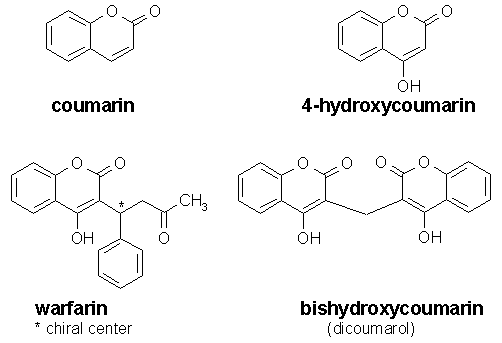
Related compounds and derivatives
Compounds derived from coumarin are also called coumarins or coumarinoids; this family includes:
Although coumarin itself has no anticoagulant properties, it is transformed into the natural anticoagulant dicoumarol by a number of species of fungi. This occurs as the result of the production of 4-hydroxycoumarin, then further (in the presence of naturally occurring formaldehyde) into the actual anticoagulant dicoumarol, a fermentation product and mycotoxin. Dicoumarol substance was responsible for the bleeding disease known historically as "sweet clover disease" in cattle eating moldy sweet clover silage. Coumarin has anti-tumor activity and anti-fungal properties.
Use as pesticides
Many of the above-stated compounds (to be specific, the 4-hydroxycoumarins, sometimes loosely called coumarins) are used as anticoagulant drugs and/or as rodenticides, which work by the anticoagulant mechanism. They block the regeneration and recycling of vitamin K. These chemicals are sometimes also incorrectly referred to as "coumadins" rather than 4-hydroxycoumarins. Coumadin is a brand name for the drug warfarin.
Some of the 4-hydroxycoumarin anticoagulant class of chemicals are designed to have very high potency and long residence times in the body, and these are used specifically as poison rodenticides. Death occurs after a period of several days to two weeks, usually from internal hemorrhaging.
Vitamin K is a true antidote for poisoning by these antirodenticide 4-hydroxycoumarins such as bromadiolone. Treatment usually comprises a large dose of vitamin K given intravenously immediately, followed by doses in pill form for a period of at least two weeks, though usually three to four, afterwards. Treatment may even continue for several months. If caught early, the prognosis is good, even when large amounts are ingested. In the short term, transfusion with fresh frozen plasma to provide clotting factors, provides time for vitamin K to reverse enzyme poisoning in the liver, and allow new clotting factors to be synthesized there.
