 | ||
The bicarbonate buffer system is an acid-base homeostatic mechanism involving the balance of carbonic acid (H2CO3), bicarbonate ion (HCO−
3), and carbon dioxide (CO2) in order to maintain pH in the blood and duodenum, among other tissues, to support proper metabolic function. Catalyzed by carbonic anhydrase, carbon dioxide (CO2) reacts with water (H2O) to form carbonic acid (H2CO3), which in turn rapidly dissociates to form a bicarbonate ion (HCO−
3 ) and a hydrogen ion (H+) as shown in the following reaction:
Contents
- In systemic acidbase balance
- Regulation
- HendersonHasselbalch equation
- Derivation of the Kassirer Bleich approximation
- In other tissues
- References
As with any buffer system, the pH is balanced by the presence of both a weak acid (for example, H2CO3) and its conjugate base (for example, HCO−
3) so that any excess acid or base introduced to the system is neutralized.
Failure of this system to function properly results in acid-base imbalance such as acidemia (pH<7.35) and alkalemia (pH>7.45) in the blood.
In systemic acid–base balance
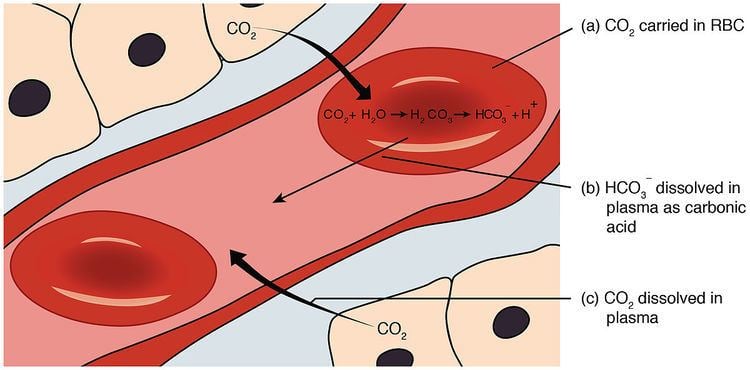
In tissue, cellular respiration produces carbon dioxide as a waste product; as one of the primary roles of the cardiovascular system, most of this CO2 is rapidly removed from the tissues by its hydration to bicarbonate ion. The bicarbonate ion present in the blood plasma is transported to the lungs, where it is dehydrated back into CO2 and released during exhalation. These hydration and dehydration conversions of CO2 and H2CO3, which are normally very slow, are facilitated by carbonic anhydrase in both the blood and duodenum. While in the blood, bicarbonate ion serves to neutralize acid introduced to the blood through other metabolic processes (e.g. lactic acid, ketone bodies); likewise, any bases (e.g. urea from the catabolism of proteins) are neutralized by carbonic acid.
Regulation
As calculated by the Henderson-Hasselbalch equation, in order to maintain a normal pH of 7.4 in the blood (whereby the pKa of carbonic acid is 6.1 at physiological temperature), a 20:1 bicarbonate to carbonic acid must constantly be maintained; this homeostasis is mainly mediated by pH sensors in the medulla oblongata of the brain and probably in the kidneys, linked via negative feedback loops to effectors in the respiratory and renal systems. In the blood of most animals, the bicarbonate buffer system is coupled to the lungs via respiratory compensation, the process by which the rate of breathing changes to compensate for changes in the blood concentration of CO2. By Le Chȃtlier’s Principle, the release of CO2 from the lungs pushes the reaction above to the left, causing carbonic anhydrase to form CO2 until all excess acid is removed. Bicarbonate concentration is also further regulated by renal compensation, the process by which the kidneys regulate the concentration of bicarbonate ions by excreting H+ ions into the urine while, at the same time, secreting HCO−
3 ions into the blood plasma, or vice versa, depending on whether the plasma pH is falling or rising, respectively.
Henderson–Hasselbalch equation
A modified version of the Henderson–Hasselbalch equation can be used to relate the pH of blood to constituents of the bicarbonate buffer system:
, where:
3] is the concentration of bicarbonate in the blood
This is useful in arterial blood gas, but these usually state pCO2, that is, the partial pressure of carbon dioxide, rather than H2CO3. However, these are related by the equation:
, where:
Taken together, the following equation can be used to relate the pH of blood to the concentration of bicarbonate and the partial pressure of carbon dioxide:
, where:
3] is the concentration of bicarbonate in the blood, in mmol/L
Derivation of the Kassirer-Bleich approximation
The Henderson Equation, which is derived from the Law of Mass Action, can be modified with respect to the bicarbonate buffer system to yield a simpler equation that provides a quick approximation of the H+ or HCO−
3 concentration without the need to calculate logarithms:
Since the partial pressure of carbon dioxide is much easier to obtain from measurement than carbonic acid, the Henry’s Law solubility constant – which relates the partial pressure of a gas to its solubility – for CO2 in plasma is used in lieu of the carbonic acid concentration. After rearranging the equation and applying Henry’s Law, the equation becomes:
Where K’ is the dissociation constant from the pKa of carbonic acid, 6.1, which is equal to 800nmol/L (since K’ = 10−pKa = 10−(6.1) ≈ 8.00X10−07mol/L = 800nmol/L).
By multiplying K’ (expressed as nmol/L) and 0.03 (800 X 0.03 = 24) and rearranging with respect to HCO−
3, the equation is simplified to:
In other tissues
The bicarbonate buffer system plays a vital role in other tissues as well. In the human stomach and duodenum, the bicarbonate buffer system serves to both neutralize gastric acid and stabilize the intracellular pH of epithelial cells via the secretion of bicarbonate ion into the gastric mucosa. In patients with duodenal ulcers, Heliobacter pylori eradication can restore mucosal bicarbonate secretion, and reduce the risk of ulcer recurrence.
