Formula C3H6O3 Molar mass 90.08 g/mol Melting point 16.8 °C | IUPAC ID 2-Hydroxypropanoic acid Boiling point 122 °C | |
 | ||
Related compounds | ||
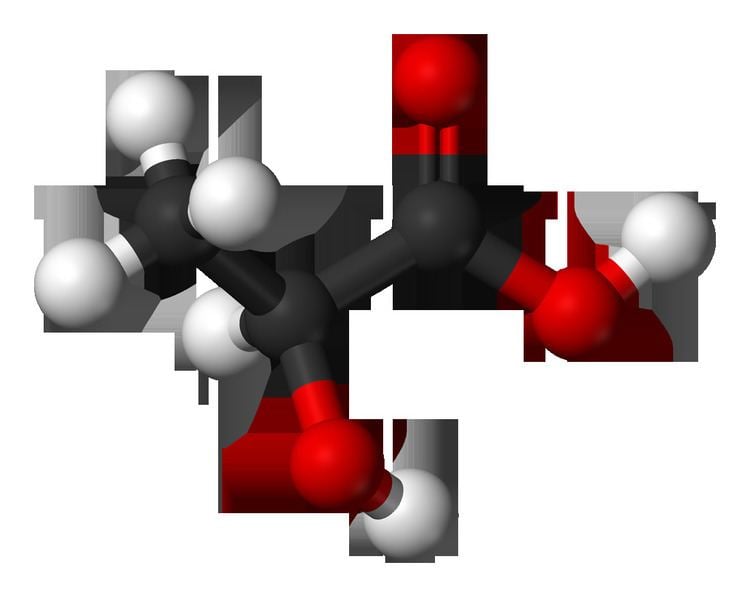
Lactic acid is an organic compound with the formula CH3CH(OH)CO2H. In its solid state, it is white and water-soluble. In its liquid state, it is clear. It is produced both naturally and synthetically. With a hydroxyl group adjacent to the carboxyl group, lactic acid is classified as an alpha-hydroxy acid (AHA). In the form of its conjugate base called lactate, it plays a role in several biochemical processes.
Contents
- History
- Production
- Exercise and lactate
- Brain metabolism
- Blood testing
- Polymer precursor
- Pharmaceutical and cosmetic applications
- Foods
- Detergents
- Mosquito lure
- Forgery
- References
In solution, it can ionize a proton from the carboxyl group, producing the lactate ion CH
3CH(OH)CO−
2. Compared to acetic acid, its pKa is 1 unit less, meaning lactic acid deprotonates ten times more easily than acetic acid does. This higher acidity is the consequence of the intramolecular hydrogen bonding between the α-hydroxyl and the carboxylate group.
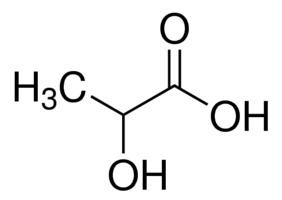
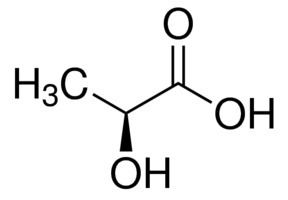
Lactic acid is chiral, consisting of two optical isomers. One is known as L-(+)-lactic acid or (S)-lactic acid and the other, its mirror image, is D-(−)-lactic acid or (R)-lactic acid. A mixture of the two in equal amounts is called DL-lactic acid, or racemic lactic acid.
Lactic acid is hygroscopic. DL-lactic acid is miscible with water and with ethanol above its melting point which is around 17 or 18 °C. D-lactic acid and L-lactic acid have a higher melting point.
In animals, L-lactate is constantly produced from pyruvate via the enzyme lactate dehydrogenase (LDH) in a process of fermentation during normal metabolism and exercise. It does not increase in concentration until the rate of lactate production exceeds the rate of lactate removal, which is governed by a number of factors, including monocarboxylate transporters, concentration and isoform of LDH, and oxidative capacity of tissues. The concentration of blood lactate is usually 1–2 mmol/L at rest, but can rise to over 20 mmol/L during intense exertion and as high as 25 mmol/L afterward.
In industry, lactic acid fermentation is performed by lactic acid bacteria, which convert simple carbohydrates such as glucose, sucrose, or galactose to lactic acid. These bacteria can also grow in the mouth; the acid they produce is responsible for the tooth decay known as caries.
In medicine, lactate is one of the main components of lactated Ringer's solution and Hartmann's solution. These intravenous fluids consist of sodium and potassium cations along with lactate and chloride anions in solution with distilled water, generally in concentrations isotonic with human blood. It is most commonly used for fluid resuscitation after blood loss due to trauma, surgery, or burns.
History
Lactic acid was isolated for the first time by the Swedish chemist Carl Wilhelm Scheele in 1780 from sour milk. The name reflects the lact- combining form derived from the Latin word for milk. In 1808, Jöns Jacob Berzelius discovered that lactic acid (actually L-lactate) also is produced in muscles during exertion. Its structure was established by Johannes Wislicenus in 1873.
In 1856, Louis Pasteur discovered Lactobacillus and its role in the making of lactic acid. Lactic acid started to be produced commercially by the German pharmacy Boehringer Ingelheim in 1895.
In 2006, global production of lactic acid reached 275,000 tonnes with an average annual growth of 10%.
Production
Lactic acid is produced industrially by bacterial fermentation of carbohydrates (sugar, starch) or by chemical synthesis from acetaldehyde, that is available from coal or crude oil. In 2009 the lactic acid was produced predominantly by fermentation (70-90 %). By microbial fermentation is possible to produce racemic lactic acid (50:50 mixture of D and L stereoisomers) or mixtures with up to 99.9% L-lactic acid. Production of D-lactic acid on industrial scale by fermentation is possible, but is much more challenging.
Fermentative production
Fermented milk products are obtained industrially by fermentation of milk or whey by Lactobacillius-species: Lactobacillus acidophilus, Lactobacillus casei, Lactobacillus delbrueckii subsp. bulgaricus (Lactobacillus bulgaricus) and Lactobacillus helveticus, and furthermore Streptococcus salivarius subsp. thermophilus (Streptococcus thermophilus) and Lactococcus lactis.
As a starting material for industrial production of lactic chemistry, that is applied for chemical synthesis, almost any carbohydrate source containing C5/C6 sugars could be used. Pure sucrose, glucose from starch, raw sugar beet juice are frequently applied. Lactic acid producing bacteria could be divided in two classes: homofermentative bacteria like Lactobacillus casei and Lactococcus lactis, producing two moles of lactate fron one mole of glucose, heterofermentative species producing one mole of lactate from one mole of glucose as well as carbon dioxide and acetic acid/ethanol.
Chemical production
Racemic lactic acid is produced in industry by addition of hydrogen cyanide to acetaldehyde and subsequent hydrolysis of forming lactonitrile. Hydrolysis performed by hydrochloric acid and ammonium chloride forms as a by-product. Japanese concern Musashino is one of the last big manufactures of lactic acid by this route. Synthesis of both racemic and enantiopure lactic acids is also possible from other starting materials (vinyl acetate, glycerol, etc.) by application of catalytic procedures.
Exercise and lactate
During power exercises such as sprinting, when the rate of demand for energy is high, glucose is broken down and oxidized to pyruvate, and lactate is then produced from the pyruvate faster than the body can process it, causing lactate concentrations to rise. The production of lactate is beneficial because it regenerates NAD+ (pyruvate is reduced to lactate while NADH is oxidized to NAD+), which is used up in oxidation of glyceraldehyde 3-phosphate during production of pyruvate from glucose, and this ensures that energy production is maintained and exercise can continue. (During intense exercise, the respiratory chain cannot keep up with the amount of hydrogen atoms that join to form NADH, and cannot regenerate NAD+ quickly enough.)
The resulting lactate can be used in two ways:
However, lactate is continually formed even at rest and during moderate exercise. Some causes of this are metabolism in red blood cells that lack mitochondria, and limitations resulting from the enzyme activity that occurs in muscle fibers having a high glycolytic capacity.
In 2004 Robergs et al. maintained that lactic acidosis during exercise is a "construct" or myth, pointing out that part of the H+ comes from ATP hydrolysis (ATP4− + H2O → ADP3− + HPO2−
4 + H+), and that reducing pyruvate to lactate (pyruvate− + NADH + H+ → lactate− + NAD+) actually consumes H+. Lindinger et al. countered that they had ignored the causative factors of the increase in [H+]. After all, the production of lactate− from a neutral molecule must increase [H+] to maintain electroneutrality. The point of Robergs's paper, however, was that lactate− is produced from pyruvate−, which has the same charge. It is pyruvate− production from neutral glucose that generates H+:
Although the reaction glucose → 2 lactate− + 2 H+ releases two H+ when viewed on its own, the H+ are absorbed in the production of ATP. On the other hand, the absorbed acidity is released during subsequent hydrolysis of ATP: ATP4− + H2O → ADP3− + HPO2−
4 + H+. So once the use of the ATP is included, the overall reaction is
3COCO−
2 + 2 H+
The generation of CO2 during respiration also causes an increase in [H+].
Brain metabolism
Although glucose is usually assumed to be the main energy source for living tissues, there are some indications that it is lactate, and not glucose, that is preferentially metabolized by neurons in the brain of several mammalian species (the notable ones being mice, rats, and humans). According to the lactate-shuttle hypothesis, glial cells are responsible for transforming glucose into lactate, and for providing lactate to the neurons. Because of this local metabolic activity of glial cells, the extracellular fluid immediately surrounding neurons strongly differs in composition from the blood or cerebro-spinal fluid, being much richer with lactate, as was found in microdialysis studies.
Some evidence suggests that lactate is important at early stages of development for brain metabolism in prenatal and early postnatal subjects, with lactate at these stages having higher concentrations in body liquids, and being utilized by the brain preferentially over glucose. It was also hypothesized that lactate may exert a strong action over GABAergic networks in the developing brain, making them more inhibitory than it was previously assumed, acting either through better support of metabolites, or alterations in base intracellular pH levels, or both.
Studies of brain slices of mice show that beta-hydroxybutyrate, lactate, and pyruvate act as oxidative energy substrates, causing an increase in the NAD(P)H oxidation phase, that glucose was insufficient as an energy carrier during intense synaptic activity and, finally, that lactate can be an efficient energy substrate capable of sustaining and enhancing brain aerobic energy metabolism in vitro. The study "provides novel data on biphasic NAD(P)H fluorescence transients, an important physiological response to neural activation that has been reproduced in many studies and that is believed to originate predominately from activity-induced concentration changes to the cellular NADH pools."
Blood testing
Blood tests for lactate are performed to determine the status of the acid base homeostasis in the body. Blood sampling for this purpose is often by arterial blood sampling (even if it is more difficult than venipuncture), because lactate differs substantially between arterial and venous levels, and the arterial level is more representative for this purpose.
During childbirth, lactate levels in the fetus can be quantified by fetal scalp blood testing.
Polymer precursor
Two molecules of lactic acid can be dehydrated to the lactone lactide. In the presence of catalysts lactide polymerize to either atactic or syndiotactic polylactide (PLA), which are biodegradable polyesters. PLA is an example of a plastic that is not derived from petrochemicals.
Pharmaceutical and cosmetic applications
Lactic acid is also employed in pharmaceutical technology to produce water-soluble lactates from otherwise-insoluble active ingredients. It finds further use in topical preparations and cosmetics to adjust acidity and for its disinfectant and keratolytic properties.
Foods
Lactic acid is found primarily in sour milk products, such as koumiss, laban, yogurt, kefir, some cottage cheeses, and kombucha. The casein in fermented milk is coagulated (curdled) by lactic acid. Lactic acid is also responsible for the sour flavor of sourdough breads.
Often in lists of nutritional information, such as the USDA National Nutrient Database, lactic acid will be included under the term "carbohydrate" (or "carbohydrate by difference") because this includes everything other than water, protein, fat, ash, and ethanol. This means a value of 4 calories per gram will be used for any lactic acid in calculating the food energy.
In beer brewing some styles of beer (sour beer) purposely contain lactic acid. Most commonly this is produced naturally by various strains of bacteria. These bacteria ferment sugars into acids, unlike yeast, who ferment sugar into ethanol. One such style are Belgian Lambics. After cooling the wort, yeast and bacteria are allowed to “fall” into the open fermenters. Most brewers of more common beer styles would ensure no such bacteria are allowed to enter the fermenter. Other sour styles of beer include Berliner weisse, Flanders red and American wild ale.
In winemaking, a bacterial process, natural or controlled, is often used to convert the naturally present malic acid to lactic acid, to reduce the sharpness and for other flavor-related reasons. This malolactic fermentation is undertaken by the family of lactic acid bacteria.
As a food additive it is approved for use in the EU, USA and Australia and New Zealand; it is listed by its INS number 270 or as E number E270. Lactic acid is used as a food preservative, curing agent, and flavoring agent. It is an ingredient in processed foods and is used as a decontaminant during meat processing. Lactic acid is produced commercially by fermentation of carbohydrates such as glucose, sucrose, or lactose, or by chemical synthesis. Carbohydrate sources include corn, beets, and cane sugar.
Detergents
Lactic acid has gained importance in the detergent industry the last decade. It is a good descaler, soap-scum remover, and a registered anti-bacterial agent. It is also economically beneficial as well as part of a trend toward environmentally safer and natural ingredients.
Mosquito lure
Lactic acid, along with ammonium bicarbonate, is used in the Lurex brand mosquito attractant.
Forgery
Lactic acid has historically been used to assist with the erasure of inks from official papers to be modified during forgery.
