ATC code V03AB22 (WHO) PubChem CID 10026 ChemSpider 9632 | CAS Number 110-46-3 DrugBank DB01612 | |
 | ||
Synonyms Isoamyl nitrite,Nitramyl,3-methyl-1-nitrosooxybutane,Pentyl alcohol nitrite (ambiguous),poppers (colloquial, slang) | ||
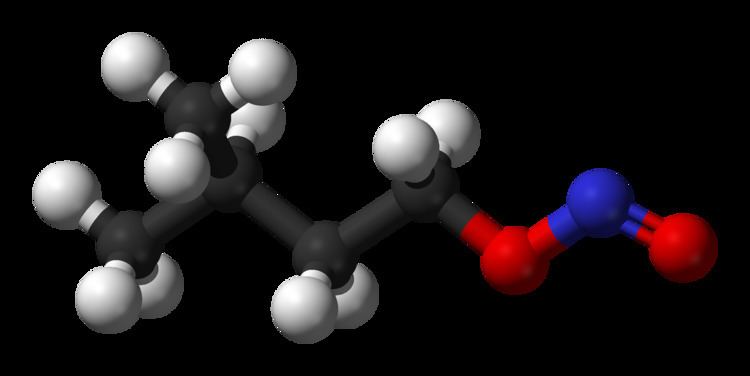
Amyl nitrite is a chemical compound with the formula C5H11ONO. A variety of isomers are known, but they all feature an amyl group attached to the nitrite functional group. The alkyl group is unreactive and the chemical and biological properties are mainly due to the nitrite group. Like other alkyl nitrites, amyl nitrite is bioactive in mammals, being a vasodilator, which is the basis of its use as a prescription medicine. As an inhalant, it also has a psychoactive effect, which has led to its recreational use with its smell being described as that of old socks or dirty feet. It is also referred to as banapple gas.
Contents
Uses
Nomenclature
The term "amyl nitrite" encompasses several isomers. For example, a common form of amyl nitrite with the formula (CH3)2CHCH2CH2ONO may be more specifically referred to as isoamyl nitrite. When the amyl group is a linear or normal (n) alkyl group, the resulting amyl nitrite would have the structural formula CH3(CH2)4ONO.
Despite a very similar name to amyl nitrite, amyl nitrate has a different chemical composition and different properties. Vast numbers of people confuse the two drugs, especially those wishing to purchase "poppers" (amyl nitrite) for recreational purposes.
Synthesis and reactions
Alkyl nitrites are prepared by the reaction of alcohols with nitrous acid:
C5H11OH + HONO → C5H11ONO + H2OThe reaction is called esterification. Synthesis of alkyl nitrites is, in general, straightforward and can be accomplished in home laboratories. A common procedure includes the dropwise addition of concentrated sulfuric acid to a cooled mixture of an aqueous sodium nitrite solution and an alcohol. The intermediately-formed stoichiometric mixture of nitrous and nitric oxide then converts the alcohol to the alkyl nitrite, which, due to its low density, will form an upper layer that can be easily decanted from the reaction mixture.
Isoamyl nitrite decomposes in the presence of base to give nitrite salts and the isoamyl alcohol:
C5H11ONO + NaOH → C5H11OH + NaNO2Amyl nitrite, like other alkyl nitrites, reacts with carbanions to give oximes.
Amyl nitrites are also useful as reagents in a modification of the Sandmeyer reaction. The reaction of the alkyl nitrite with an aromatic amine in a halogenated solvent produces a radical aromatic species, this then frees a halogen atom from the solvent. For the synthesis of aryl iodides diiodomethane is used, whereas bromoform is the solvent of choice for the synthesis of aryl bromides.
Physiological effects
Amyl nitrite, in common with other alkyl nitrites, is a potent vasodilator; it expands blood vessels, resulting in lowering of the blood pressure. Alkyl nitrites are a source of nitric oxide, which signals for relaxation of the involuntary muscles. Physical effects include decrease in blood pressure, headache, flushing of the face, increased heart rate, dizziness, and relaxation of involuntary muscles, especially the blood vessel walls and the internal and external anal sphincter. There are no withdrawal symptoms. Overdose symptoms include nausea, vomiting, hypotension, hypoventilation, shortness of breath, and fainting. The effects set in very quickly, typically within a few seconds and disappear within a few minutes. Amyl nitrite may also intensify the experience of synesthesia.
