Organic Chemistry Portal sandmeyer-reaction | ||
 | ||
The Sandmeyer reaction is a chemical reaction used to synthesize aryl halides from aryl diazonium salts. It is named after the Swiss chemist Traugott Sandmeyer. The reaction is a method for substitution of an aromatic amino group via preparation of its diazonium salt followed by its displacement with a nucleophile, often catalyzed by copper(I) salts. The nucleophile can include halide anions, cyanide, thiols, water, and others. The reaction does not proceed well with the fluoride anion, but fluorination can be carried out using tetrafluoroborate anions (Balz–Schiemann reaction).
Contents
Reaction mechanism
The nitrous acid is usually prepared in situ from sodium nitrite and an acid. Following a 2nd protonation step, one equivalent of water is lost to form nitrogen monoxide cation i.e. the "nitrosonium ion" electrophile.
An aromatic (or heterocyclic) amine quickly reacts with a nitrite to form an aryl diazonium salt, which decomposes in the presence of copper(I) salts, such as CuCl, to form the desired aryl halide. It is carried out at the temperature of 25-30 °C. The reaction is a radical-nucleophilic aromatic substitution.
Several improvements have been made to the standard procedures.
Variations
The majority of variations of the Sandmeyer reactions consist of using various copper salts. For example, using cuprous cyanide produces benzonitriles. Substituting thiols or water for the copper salts generates thioethers or phenols, respectively.
The Schiemann reaction uses tetrafluoroborate and delivers the halide-substituted product, fluorobenzene, which is not obtained by the use of copper fluorides.
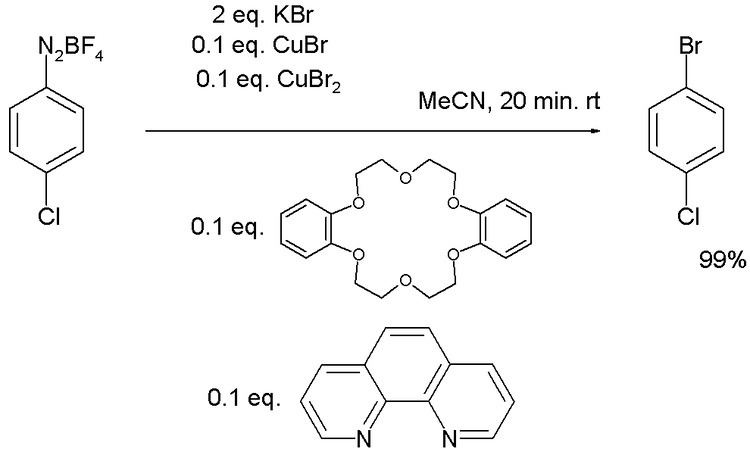
Sandmeyer reactions with copper salts used in catalytic amounts are also known. One bromination protocol employs a 0.2 equivalent Cu(I)/Cu(II) mixture with additional amounts of the bidentate ligand phenanthroline and phase-transfer catalyst dibenzo-18-crown-6:
Amyl nitrites are also useful as reagents in a modification of the Sandmeyer reaction. The reaction of the alkyl nitrite with an aromatic amine in a halogenated solvent produces a radical aromatic species, this then abstracts a halogen atom from the solvent. For the synthesis of aryl iodides diiodomethane is used, whereas bromoform is the solvent of choice for the synthesis of aryl bromides.
Aliphatic variation
William Reusch at Michigan State University states:
The aliphatic version of the Sandmeyer reaction was used to prepare Batimastat and marimastat though (from 1° amino acids).
