Related compounds Molar mass 568.88 g/mol Appearance orange-red | Formula C40H56O2 Classification Xanthophyll | |
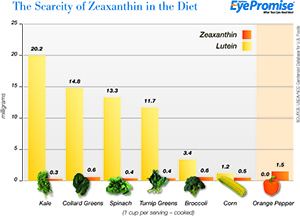 | ||
You need lutein and zeaxanthin
Zeaxanthin is one of the most common carotenoid alcohols found in nature. It is important in the xanthophyll cycle. Synthesized in plants and some micro-organisms, it is the pigment that gives paprika (made from bell peppers), corn, saffron, wolfberries, and many other plants and microbes their characteristic color.
Contents
- You need lutein and zeaxanthin
- Isomers and macular uptake
- Relationship with diseases of the eye
- Natural occurrence
- Safety
- References
The name (pronounced zee-uh-zan'-thin) is derived from Zea mays (common yellow maize corn, in which zeaxanthin provides the primary yellow pigment), plus xanthos, the Greek word for "yellow" (see xanthophyll).
Xanthophylls such as zeaxanthin are found in highest quantity in the leaves of most green plants, where they act to modulate light energy and perhaps serve as a non-photochemical quenching agent to deal with triplet chlorophyll (an excited form of chlorophyll) which is overproduced at high light levels during photosynthesis.
Animals derive zeaxanthin from a plant diet. Zeaxanthin is one of the two primary xanthophyll carotenoids contained within the retina of the eye. Within the central macula, zeaxanthin is the dominant component, whereas in the peripheral retina, lutein predominates.
Zeaxanthin supplements are typically taken on the supposition of supporting eye health. Although there are no reported side effects from taking zeaxanthin supplements, this possible benefit remains scientifically unproven, despite extensive ongoing research to define dietary or supplemental effects of zeaxanthin and lutein.
As a food additive, zeaxanthin is a food dye with E number E161h.
Isomers and macular uptake
Lutein and zeaxanthin have identical chemical formulas and are isomers, but they are not stereoisomers. The only difference between them is in the location of the double bond in one of the end rings. This difference gives lutein three chiral centers whereas zeaxanthin has two. Because of symmetry, the (3R,3'S) and (3S,3'R) stereoisomers of zeaxanthin are identical. Therefore, zeaxanthin has only three stereoisomeric forms. The (3R,3'S) stereoisomer is called meso-zeaxanthin.
The principal natural form of zeaxanthin is (3R,3'R)-zeaxanthin. The macula mainly contains the (3R,3'R)- and meso-zeaxanthin forms, but it also contains much smaller amounts of the third (3S,3'S) form. Evidence exists that a specific zeaxanthin-binding protein recruits circulating zeaxanthin and lutein for uptake within the macula.
Due to the commercial value of carotenoids, their biosynthesis has been studied extensively in both natural products and non-natural (heterologous) systems such as the bacteria Escherichia coli and yeast Saccharomyces cerevisiae. Zeaxanthin biosynthesis proceeds from beta-carotene via the action of a single protein, known as a beta-carotene hydroxylase, that is able to add a hydroxyl group (-OH) to carbon 3 and 3' of the beta-carotene molecule. Zeaxanthin biosynthesis therefore proceeds from beta-carotene to zeaxanthin (a di-hydroxylated product) via beta-cryptoxanthin (the mono hydroxylated intermediate). Although functionally identical, several distinct beta-carotene hydroxylase proteins are known. Due to the nature of zeaxanthin, relative to astaxanthin (a carotenoid of significant commercial value) beta-carotene hydroxylase proteins have been studied extensively.
Relationship with diseases of the eye
Several observational studies have provided preliminary evidence for high dietary intake of foods including lutein and zeaxanthin with lower incidence of age-related macular degeneration (AMD), most notably the Age-Related Eye Disease Study (AREDS2). Because foods high in one of these carotenoids tend to be high in the other, research does not separate effects of one from the other. Three subsequent meta-analyses of dietary lutein and zeaxanthin all conclude that these carotenoids lower the risk of progression from early stage AMD to late stage AMD. In general, however, there remains insufficient evidence to assess the effectiveness of dietary or supplemental zeaxanthin or lutein in treatment or prevention of early AMD
As for cataracts, two meta-analyses confirm a correlation between high serum concentrations of lutein and zeaxanthin and a decrease in the risk of nuclear cataract, but not cortical or subcapsular cataract. The reports did not separate zeaxanthin effect from lutein effect. There is only one published clinical intervention trial testing for an effect of lutein and zeaxanthin supplementation on cataracts. The AREDS2 trial enrolled subjects at risk for progression to advanced age-related macular degeneration. Overall, the group getting lutein (10 mg) and zeaxanthin (2 mg) were NOT less likely to progress to needed cataract surgery. The authors speculated that there may be a cataract prevention benefit for people with low dietary intake of lutein and zeaxanthin, but recommended more research. Any benefit is more likely to be apparent in subpopulations of individuals exposed to high oxidative stress, such as heavy smokers, alcoholics or those with low dietary intake of carotenoid-rich foods.
In 2005, the US Food and Drug Administration rejected a Qualified Health Claims application by Xangold, citing insufficient evidence supporting the use of a lutein- and zeaxanthin-containing supplement in prevention of AMD. Although more convincing evidence has accrued, no company had reapplied for a label health claim. Dietary supplement companies in the U.S. are allowed to sell lutein and lutein + zeaxanthin products using Structure:Function language such as "Helps maintain eye health" as long as the FDA disclaimer statement ("These statements have not been evaluated...") is on the label. In Europe, as recently as 2014, the European Food Safety Authority reviewed and rejected claims that lutein or lutein plus zeaxanthin improved vision.
Natural occurrence
Zeaxanthin is the pigment that gives paprika (made from bell peppers), corn, saffron, wolfberries, and many other plants their characteristic color. Spirulina is also a rich source and can serve as a dietary supplement. Zeaxanthin breaks down to form picrocrocin and safranal, which are responsible for the taste and aroma of saffron.
Foods containing the highest amounts of lutein and zeaxanthin are dark green leaf vegetables, such as kale, spinach, turnip greens, collard greens, romaine lettuce, watercress, Swiss chard and mustard greens.
Safety
An acceptable daily intake level for zeaxanthin was proposed as 0.75 mg/kg of body weight/day, or 53 mg/day for a 70 kg adult. In humans, an intake of 20 mg/day for up to six months had no adverse effects. As of 2016, neither the U.S. Food and Drug Administration nor the European Food Safety Authority have set a Tolerable Upper Intake Level (UL) for lutein or zeaxanthin.
