 | ||
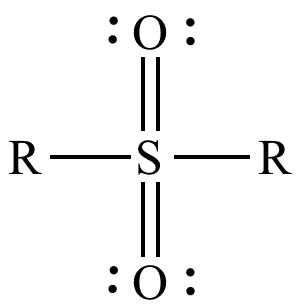
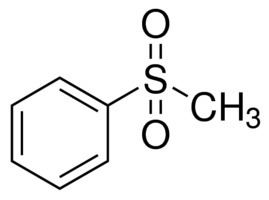
A sulfone is a chemical compound containing a sulfonyl functional group attached to two carbon atoms. The central hexavalent sulfur atom is double bonded to each of two oxygen atoms and has a single bond to each of two carbon atoms, usually in two separate hydrocarbon substituents.
Contents
Synthesis
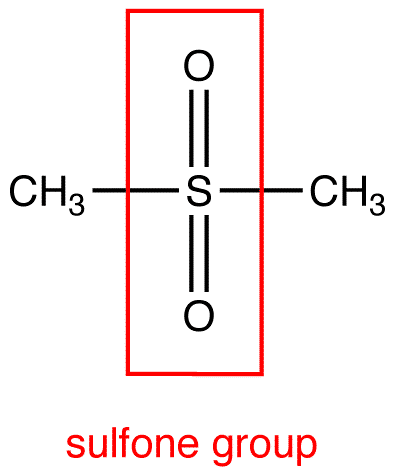
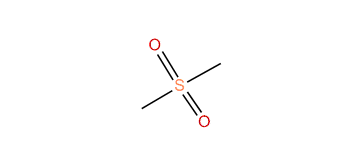
The general structural formula is R–S(=O)2–R′ where R and R′ are the organic groups. Sulfides are often the precursors to sulfones by organic oxidation through the intermediate formation of sulfoxides. For example, dimethyl sulfide is oxidized to dimethyl sulfoxide and then to dimethyl sulfone. In the Ramberg-Bäcklund Reaction and the Julia olefination sulfones are converted to alkenes through the elimination of sulfur dioxide.
The industrially useful sulfone is sulfolane, a cyclic molecule with the formula (CH2)4SO2. It is typically prepared, not by oxidation of the thioether, but by addition of sulfur dioxide to 1,3-butadiene, followed by hydrogenation of the resulting sulfolene.
Solvents
Sulfolane is probably the sulfone produced on the largest scale. It is used to extract valuable aromatic compounds ("BTX") from petroleum.
Polymers
Some polymers containing sulfone groups have gained prominence in the field of engineering plastics. Various materials exhibit high strength and resistance to oxidation, corrosion, high temperatures, and creep under stress. For example, some are valuable as replacements for copper in domestic hot water plumbing. Precursors to such polymers are the sulfones bisphenol S and 4,4'-dichlorodiphenyl sulfone.
Pharmacology
Examples of sulfones in pharmacology include dapsone, a drug formerly used as an antibiotic to treat leprosy, dermatitis herpetiformis, tuberculosis, or pneumocystis pneumonia (PCP). Several of its derivatives, such as promin, have similarly been studied or actually been applied in medicine, but in general sulfones are of far less prominence in pharmacology than for example the sulfonamides.
Nomenclature
In the literature, particularly lay literature, sulfones commonly are confused with sulfonamides, though the latter have one carbon and one nitrogen atom attached to the sulfur atom, instead of two carbon atoms. The pharmacological mechanisms accordingly differ from that of the sulfonamides. However, in practice one commonly sees frequent references to dapsone and promin as sulfonamides. Probably this is partly because few pharmaceuticals are in fact sulfones. The use of the long-standing alternative spelling sulphone is discouraged by IUPAC; it is definitely undesirable to have two spellings in simultaneous common use, and it was agreed to discontinue the ph spelling as the more archaic.
Selenones
Selenketones and tellurones are the selenium and tellurium versions of the sulfones, though they are more reactive.
