Pronunciation /ˈlɛprəsi/ ICD-10 A30 OMIM 246300 | Specialty Infectious disease ICD-9-CM 030 DiseasesDB 8478 | |
 | ||
Leprosy, also known as Hansen's disease (HD), is a long-term infection by the bacteria Mycobacterium leprae or Mycobacterium lepromatosis. Initially, infections are without symptoms and typically remain this way for 5 to 20 years. Symptoms that develop include granulomas of the nerves, respiratory tract, skin, and eyes. This may result in a lack of ability to feel pain, thus loss of parts of extremities due to repeated injuries or infection due to unnoticed wounds. Weakness and poor eyesight may also be present.
Contents
- Signs and symptoms
- M leprae
- Risk factors
- Transmission
- Genetics
- Pathophysiology
- Diagnosis
- Classification
- Prevention
- Treatment
- Epidemiology
- Disease burden
- History
- India
- Treatment cost
- Historical texts
- Middle Ages
- Norway
- Colonialism and imperialism
- Stigma
- Programs and treatment
- Notable cases
- Other animals
- References
Leprosy is spread between people. This is thought to occur through a cough or contact with fluid from the nose of an infected person. Leprosy occurs more commonly among those living in poverty. Contrary to popular belief, it is not highly contagious. The two main types of disease are based on the number of bacteria present: paucibacillary and multibacillary. The two types are differentiated by the number of poorly pigmented, numb skin patches present, with paucibacillary having five or fewer and multibacillary having more than five. The diagnosis is confirmed by finding acid-fast bacilli in a biopsy of the skin or by detecting the DNA using polymerase chain reaction.
Leprosy is curable with a treatment known as multidrug therapy. Treatment for paucibacillary leprosy is with the medications dapsone and rifampicin for six months. Treatment for multibacillary leprosy consists of rifampicin, dapsone, and clofazimine for 12 months. A number of other antibiotics may also be used. These treatments are provided free of charge by the World Health Organization. Globally in 2012, the number of chronic cases of leprosy was 189,000, down from some 5.2 million in the 1980s. The number of new cases was 230,000. Most new cases occur in 16 countries, with India accounting for more than half. In the past 20 years, 16 million people worldwide have been cured of leprosy. About 200 cases are reported per year in the United States.
Leprosy has affected humanity for thousands of years. The disease takes its name from the Latin word lepra, which means "scaly", while the term "Hansen's disease" is named after the physician Gerhard Armauer Hansen. Separating people by placing them in leper colonies still occurs in places such as India, China, and Africa. However, most colonies have closed, since leprosy is not very contagious. Social stigma has been associated with leprosy for much of history, which continues to be a barrier to self-reporting and early treatment. Some consider the word "leper" offensive, preferring the phrase "person affected with leprosy". World Leprosy Day was started in 1954 to draw awareness to those affected by leprosy.
Signs and symptoms
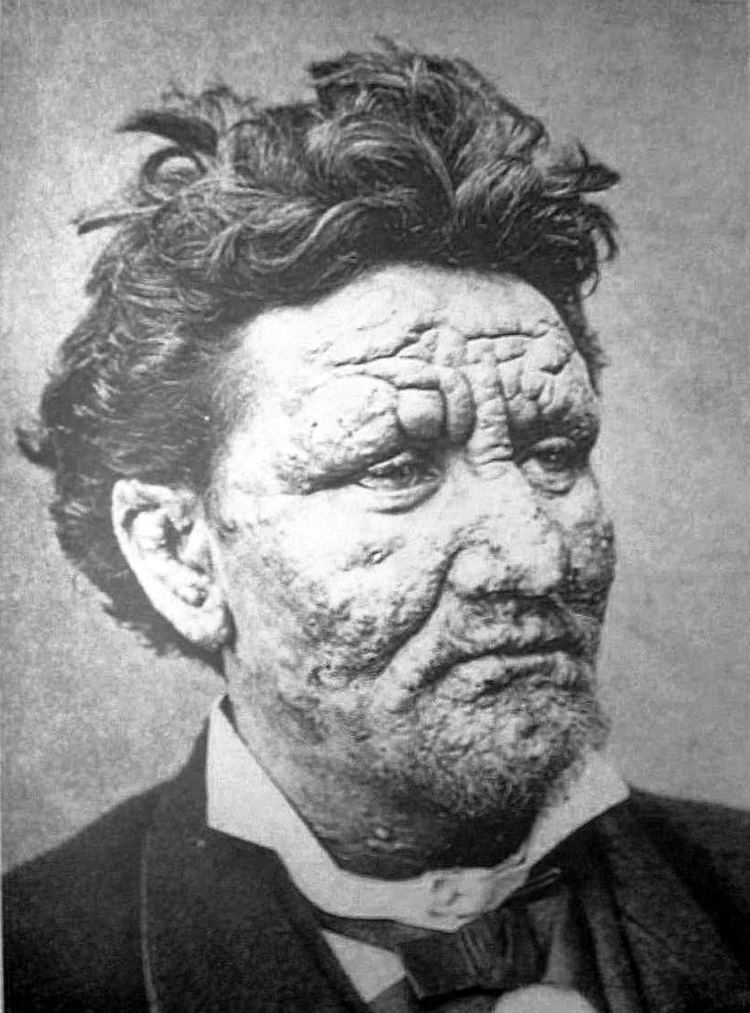
Leprosy is mostly a granulomatous disease of the peripheral nerves and mucosa of the upper respiratory tract; skin lesions (light or dark patches) are the primary external sign. If untreated, leprosy can progress and cause permanent damage to the skin, nerves, limbs, and eyes. Contrary to folklore, leprosy does not cause body parts to fall off, although they can become numb or diseased as a result of secondary infections; these occur as a result of the body's defenses being compromised by the primary disease. Secondary infections, in turn, can result in tissue loss, causing fingers and toes to become shortened and deformed, as cartilage is absorbed into the body.
M. leprae
M. leprae and M. lepromatosis are the causative agents of leprosy. M. lepromatosis is a relatively newly identified mycobacterium isolated from a fatal case of diffuse lepromatous leprosy in 2008.
An intracellular, acid-fast bacterium, M. leprae is aerobic and rod-shaped, and is surrounded by the waxy cell membrane coating characteristic of the Mycobacterium genus.
Due to extensive loss of genes necessary for independent growth, M. leprae and M. lepromatosis are obligate intracellular pathogens, and unculturable in the laboratory, a factor that leads to difficulty in definitively identifying the organism under a strict interpretation of Koch's postulates. The use of nonculture-based techniques such as molecular genetics has allowed for alternative establishment of causation.
While the causative organisms have to date been impossible to culture in vitro, it has been possible to grow them in animals such as mice and armadillos.
Naturally occurring infection also has been reported in nonhuman primates, including the African chimpanzee, sooty mangabey, and cynomolgus macaque, as well as in armadillos and red squirrels.
Red squirrels (Sciurus vulgaris) - a threatened species - in England were found to have leprosy in November 2016. No squirrel cases have spread to a human for hundreds of years, though.
Risk factors
The greatest risk factor for developing leprosy is contact with another case of leprosy. Contacts of people with leprosy are five to eight times more likely to develop leprosy than members of the general population. Other risk factors are poorly understood. However, conditions that reduce immune function, such as malnutrition, other illnesses, or host genetic differences, may increase the risk of developing leprosy. Despite this, infection with HIV does not appear to increase the risk of developing leprosy.
Transmission
Transmission of leprosy occurs during close contact with those who are infected. Transmission is proposed to be by nasal droplets, but many questions remain about its mode of transmission and epidemiology.
Leprosy is not known to be either sexually transmitted or highly infectious. People are generally no longer infectious after the first month of standard multidrug therapy.
Leprosy may also be transmitted to humans by armadillos.
Two exit routes of M. leprae from the human body often described are the skin and the nasal mucosa, although their relative importance is not clear. Lepromatous cases show large numbers of organisms deep in the dermis, but whether they reach the skin surface in sufficient numbers is doubtful.
The skin and the upper respiratory tract are most likely entry route. While older research dealt with the skin route, recent research has increasingly favored the respiratory route. Experimental transmission of leprosy through aerosols containing M. leprae in immunosuppressed mice was accomplished, suggesting a similar possibility in humans.
Genetics
Several genes have been associated with a susceptibility to leprosy. Often, the immune system is able to eliminate leprosy during the early infection stage before severe symptoms develop. A defect in cell-mediated immunity may cause susceptibility to leprosy. The region of DNA responsible for this variability is also involved in Parkinson's disease, giving rise to current speculation that the two disorders may be linked in some way at the biochemical level. Some evidence indicates not all people who are infected with M. leprae develop leprosy, and genetic factors have long been thought to play a role, due to the observation of clustering of leprosy around certain families, and the failure to understand why certain individuals develop lepromatous leprosy while others develop other types of leprosy.
Pathophysiology
How the infection produces the symptoms of the disease is not known.
Diagnosis
According to the World Health Organization, diagnosis in areas where people are frequently infected is based on one of these main signs:
Skin lesions can be single or multiple, and usually hypopigmented, although occasionally reddish or copper-colored. The lesions may be macules (flat), papules (raised), or nodular. The sensory loss at the skin lesion is important because this feature can help differentiate it from other causes of skin lesions such as tinea versicolor. Thickened nerves are associated with leprosy and can be accompanied by loss of sensation or muscle weakness. However, without the characteristic skin lesion and sensory loss, muscle weakness is not considered a reliable sign of leprosy.
In some cases, acid-fast leprosy bacilli in skin smears are considered diagnostic; however, the diagnosis is clinical.
Diagnosis in areas where the disease is uncommon, such as the United States, is often delayed because healthcare providers are unaware of leprosy and its symptoms. Early diagnosis and treatment prevent nerve involvement, the hallmark of leprosy, and the disability it causes.
Many kinds of leprosy are known, but some symptoms are common to them, including runny nose, dry scalp, eye problems, skin lesions, muscle weakness, reddish skin, smooth, shiny, diffuse thickening of facial skin, ear, and hand, loss of sensation in fingers and toes, thickening of peripheral nerves, and flat nose due to destruction of nasal cartilage. Also, phonation and resonation of sound occur during speech. Often, atrophy of the testes with resulting impotence occurs.
Classification
Several different approaches for classifying leprosy exist, but parallels exist.
A difference in immune response to the tuberculoid and lepromatous forms is seen.
Leprosy may also be divided into:
This disease may also occur with only neural involvement, without skin lesions.
Prevention
Early detection of the disease is important, since physical and neurological damage may be irreversible even if cured. Medications can decrease the risk of those living with people with leprosy from acquiring the disease and likely those with whom people with leprosy come into contact outside the home. However, concerns are known of resistance, cost, and disclosure of a person's infection status when doing follow-up of contacts. Therefore, the WHO recommends that people who live in the same household be examined for leprosy and be treated only if symptoms are present.
The Bacillus Calmette–Guérin (BCG) vaccine offers a variable amount of protection against leprosy in addition to tuberculosis. It appears to be 26 to 41% effective (based on controlled trials) and about 60% effective based on observational studies with two doses possibly working better than one. Development of a more effective vaccine is ongoing.
Treatment
A number of leprostatic agents are available for treatment. For paucibacillary (PB or tuberculoid) cases, treatment with daily dapsone and monthly rifampicin for six months is recommended. While for multibacillary (MB or lepromatous) cases, treatment with daily dapsone and clofazimine along with monthly rifampicin for 12 months is recommended.
Multidrug therapy (MDT) remains highly effective, and people are no longer infectious after the first monthly dose. It is safe and easy to use under field conditions due to its presentation in calendar blister packs. Relapse rates remain low, and no resistance to the combined drugs is seen.
Epidemiology
In 2015, the number of cases of leprosy was about 175,000 and the number of new cases was 210,000.
As of 2013, 14 countries contain 95% of the globally reported leprosy cases. Of these, India has the greatest number of cases (59%), followed by Brazil (14%) and Indonesia (8%). Although the number of cases worldwide continues to fall, pockets of high prevalence remain in certain areas such as Brazil, South Asia (India, Nepal, Bhutan), some parts of Africa (Tanzania, Madagascar, Mozambique), and the western Pacific.
The number of cases of leprosy was in the tens of millions in the 1960s, a series of national (the International Federation of Anti-Leprosy Associations) and international (the WHO's "Global Strategy for Reducing Disease Burden Due to Leprosy") initiatives have reduced the total number and the number of new cases of the disease. In 1995, two to three million people were estimated to be permanently disabled because of leprosy.
Disease burden
Although the number of new leprosy cases occurring each year is important as a measure of transmission, it is difficult to measure due to leprosy's long incubation period, delays in diagnosis after onset of the disease, and the lack of laboratory tools to detect it in the very early stages. Instead, the registered prevalence is used. Registered prevalence is a useful proxy indicator of the disease burden, as it reflects the number of active leprosy cases diagnosed with the disease and receiving treatment with MDT at a given point in time. The prevalence rate is defined as the number of cases registered for MDT treatment among the population in which the cases have occurred, again at a given point in time.
New case detection is another indicator of the disease that is usually reported by countries on an annual basis. It includes cases diagnosed with the onset of disease in the year in question (true incidence) and a large proportion of cases with onset in previous years (termed a backlog prevalence of undetected cases).
Endemic countries also report the number of new cases with established disabilities at the time of detection, as an indicator of the backlog prevalence. Determination of the time of onset of the disease is, in general, unreliable, is very labor-intensive, and is seldom done in recording these statistics.
History
Using comparative genomics, in 2005, geneticists traced the origins and worldwide distribution of leprosy from East Africa or the Near East along human migration routes. They found four strains of M. leprae with specific regional locations. Strain 1 occurs predominantly in Asia, the Pacific region, and East Africa; strain 4, in West Africa and the Caribbean; strain 3 in Europe, North Africa, and the Americas; and strain 2 only in Ethiopia, Malawi, Nepal/north India, and New Caledonia.
On the basis of this, they offer a map of the dissemination of leprosy in the world. This confirms the spread of the disease along the migration, colonisation, and slave trade routes taken from East Africa to India, West Africa to the New World, and from Africa into Europe and vice versa.
The oldest skeletal evidence for the disease was found in the human remains from the archaeological sites of Balathal and Harappa, in India and Pakistan, respectively.
Although retrospectively identifying descriptions of leprosy-like symptoms is difficult, what appears to be leprosy was discussed by Hippocrates in 460 BC. In 1846, Francis Adams produced The Seven Books of Paulus Aegineta which included a commentary on all medical and surgical knowledge and descriptions and remedies to do with leprosy from the Romans, Greeks, and Arabs.
Interpretations of the presence of leprosy have been made on the basis of descriptions in ancient Indian (Atharva Veda and Kausika Sutra), Greek, and Middle Eastern documentary sources that describe skin afflictions.
Skeletal remains from the second millennium BC, discovered in 2009, represent the oldest documented evidence for leprosy. Located at Balathal, in Rajasthan, northwest India, the discoverers suggest that if the disease did migrate from Africa to India, during the third millennium BC "at a time when there was substantial interaction among the Indus Civilization, Mesopotamia, and Egypt, there needs to be additional skeletal and molecular evidence of leprosy in India and Africa so as to confirm the African origin of the disease." A proven human case was verified by DNA taken from the shrouded remains of a man discovered in a tomb next to the Old City of Jerusalem dated by radiocarbon methods to 1–50 AD.
The causative agent of leprosy, M. leprae, was discovered by G. H. Armauer Hansen in Norway in 1873, making it the first bacterium to be identified as causing disease in humans. The first effective treatment (promin) became available in the 1940s. In the 1950s, dapsone was introduced. The search for further effective antileprosy drugs led to the use of clofazimine and rifampicin in the 1960s and 1970s. Later, Indian scientist Shantaram Yawalkar and his colleagues formulated a combined therapy using rifampicin and dapsone, intended to mitigate bacterial resistance. MDT combining all three drugs was first recommended by the WHO in 1981. These three antileprosy drugs are still used in the standard MDT regimens.
Leprosy was once believed to be highly contagious and was treated with mercury—all of which applied to syphilis, which was first described in 1530. Many early cases thought to be leprosy could actually have been syphilis. Resistance has developed to initial treatment. Until the introduction of MDT in the early 1980s, the disease could not be diagnosed and treated successfully within the community.
Japan still has sanatoriums (although Japan's sanatoriums no longer have active leprosy cases, nor are survivors held in them by law).
The importance of the nasal mucosa in the transmission of M leprae was recognized as early as 1898 by Schäffer, in particular, that of the ulcerated mucosa.
India
India was one of the first countries to have acted against leprosy. India enacted the Leprosy Act of 1898 which institutionalized those affected and segregated them by gender to prevent reproduction. The Act was difficult to enforce but was repealed in 1983 only after MDT therapy became widely available. In 1983, the National Leprosy Elimination Programme, previously the National Leprosy Control Programme, changed its methods from surveillance to the treatment of people with leprosy. India still accounts for over half of the global disease burden.
Treatment cost
Between 1995 and 1999, the WHO, with the aid of the Nippon Foundation, supplied all endemic countries with free MDT in blister packs, channeled through ministries of health. This free provision was extended in 2000 and again in 2005, 2010 and 2015 with donations by the MDT manufacturer Novartis through the WHO. In the latest agreement signed between the company and the WHO in October 2015, the provision of free MDT by the WHO to all endemic countries will run until the end of 2020. At the national level, nongovernment organizations affiliated with the national program will continue to be provided with an appropriate free supply of this WHO-supplied MDT by the government.
Historical texts
Written accounts of leprosy date back thousands of years. Various skin diseases translated as leprosy appear in the ancient Indian text, the Atharava Veda, as early as 2000 BC. Another Indian text, the Laws of Manu (1500 BC), prohibited contact with those infected with the disease and made marriage to a person infected with leprosy punishable.
Biblically speaking, the Hebraic root tsara or tsaraath (צָרַע, --tsaw-rah' -- to be struck with leprosy, to be leprous) and the Greek (λεπρός - lepros), are of broader classification than the more narrow use of the term related to Hansen's Disease. Any progressive skin disease (a whitening or splotchy bleaching of skin, raised manifestations of scales, scabs, infections, rashes, etc.…) as well as generalized molds and surface discoloration of any clothing, leather, and/or discoloration on walls surfaces throughout homes all came under the "law of leprosy" (Leviticus 14:54-57). Ancient sources also such as the Talmud (Sifra 63) make clear that tzaraath refers to various types of lesions or stains associated with ritual impurity and occurring on cloth, leather, or houses, as well as skin. It may sometimes be a symptom of the disease described in this article but has many other causes, as well. The New Testament describes instances of Jesus healing people with leprosy (Luke 5:10), although the precise relationship between this, tzaraath, and Hansen's disease is not established.
The biblical perception that people with leprosy were unclean may be connected to a passage from Leviticus 13: 44-46, among others. Judeo-Christian belief, for some, held that leprosy was of moral consequence, and, as in many societies, early Christians believed that those affected by leprosy were being punished by God for sinful behavior. Moral associations have persisted throughout history. Pope Gregory the Great (540-604) and Isidor of Seville (560-636) considered people with the disease to be heretics.
Middle Ages
It is believed that a rise in leprosy in Europe occurred in the Middle Ages based on the increased number of hospitals created to treat leprosy patients in the 12th and 13th centuries. France alone had nearly 2,000 leprosariums during this period.
The social perception in medieval communities was generally one of fear, and those people infected with the disease were thought to be unclean, untrustworthy, and morally corrupt. People with leprosy were also often required to wear clothing that identified them as such or carry a bell announcing their presence. Segregation from mainstream society was common. The third Lateran Council of 1179 and a 1346 edict by King Edward expelled lepers from city limits. Because of the moral stigma of the disease, methods of treatment were both physical and spiritual, and leprosariums were established under the purview of the church.
Norway
Norway was the location of a progressive stance on leprosy tracking and treatment and played an influential role in European understanding of the disease. In 1832, Dr. JJ Hjort conducted the first leprosy survey, thus establishing a basis for epidemiological surveys. Subsequent surveys resulted in the establishment of a national leprosy registry to study the causes of leprosy and for tracking of the rate of infection.
Early leprosy research throughout Europe was conducted by Norwegian scientists, Daniel Cornelius Danielssen and Carl Wilhelm Boeck. Their work resulted in the establishment of the National Leprosy Research and Treatment Center. Danielssen and Boeck believed the cause of leprosy transmission was hereditary. This stance was influential in advocating for the isolation of those infected by gender to prevent reproduction.
Colonialism and imperialism
Though leprosy in Europe was again on the decline by the 1860s, Western countries embraced isolation treatment out of fear of the spread of disease from developing countries, minimal understanding of bacteriology, lack of diagnostic ability or knowledge of how contagious the disease was, and missionary activity. Growing imperialism and pressures of the industrial revolution resulted in a Western presence in countries where leprosy was endemic, namely the British presence in India. Isolation treatment methods were observed by Surgeon-Mayor Henry Vandyke Carter of the British Colony in India while visiting Norway, and these methods were applied in India with the financial and logistical assistance of religious missionaries. Colonial and religious influence and associated stigma continued to be a major factor in the treatment and public perception of leprosy in endemic developing countries until the mid-twentieth century.
Stigma
Despite effective treatment and education efforts, leprosy stigma continues to be problematic in endemic developing countries. Leprosy is most prevalent amongst impoverished or marginalized populations where social stigma is likely to be compounded by other social inequities. Fears of ostracism, loss of employment, or expulsion from family and society may contribute to a delayed diagnosis and treatment.
Folk models of belief, lack of education, and religious connotations of the disease continue to influence social perceptions of those afflicted in many parts of the world. In Brazil, for example, folklore holds that leprosy is transmitted by dogs, it is a disease associated with sexual promiscuity, and is sometimes thought to be punishment for sins or moral transgressions. Socioeconomic factors also have a direct impact. Lower-class domestic workers who are often employed by those in a higher socioeconomic class may find their employment in jeopardy as physical manifestations of the disease become apparent. Skin discoloration and darker pigmentation resulting from the disease also has social repercussions.
In extreme cases in northern India, leprosy is equated with an "untouchable" status that "often persists long after (individuals with leprosy) have been cured of the disease, creating lifelong prospects of divorce, eviction, loss of employment, and ostracism from family and social networks."
Programs and treatment
The WHO states that diagnosis and treatment with MDT are easy and effective, and a 45% decline in disease burden has occurred since MDT has become more widely available. The organization emphasizes the importance of fully integrating leprosy treatment into public health services, effective diagnosis and treatment, and access to information.
In some instances in India, community-based rehabilitation is embraced by local governments and NGOs alike. Often, the identity cultivated by a community environment is preferable to reintegration, and models of self-management and collective agency independent of NGOs and government support have been desirable and successful.
Notable cases
Other animals
Wild nine-banded armadillos (Dayspus novemcinctus) in south central United States often carry Mycobacterium leprae. This is believed to be because armadillos have such a low body temperature. Leprosy lesions appear mainly in cooler body regions such as the skin and mucous membranes of the upper respiratory tract. Because of armadillo's armor, skin lesions are hard to see. Abrasions around the eyes, nose and feet are the most common signs. Infected armadillos make up a large reservoir of M. leprae and may be a source of infection for some humans in the United States or other locations in the armadillos' home range. In armadillo leprosy, lesions did not persist at the site of entry in animals, M. leprae multiplied in macrophages at the site of inoculation and lymph nodes.
