Entrez 10280 | Ensembl ENSG00000147955 | |
 | ||
Aliases SIGMAR1, ALS16, OPRS1, SIG-1R, SR-BP, SR-BP1, SRBP, hSigmaR1, sigma1R, DSMA2, sigma non-opioid intracellular receptor 1 External IDs OMIM: 601978 MGI: 1195268 HomoloGene: 39965 GeneCards: SIGMAR1 | ||
Sigma 1 receptors
The sigma-1 receptor (σ1R), one of two sigma receptor subtypes, is a chaperone protein at the endoplasmic reticulum (ER) that modulates calcium signaling through the IP3 receptor. In humans, the σ1 receptor is encoded by the SIGMAR1 gene.
Contents
- Sigma 1 receptors
- Characteristics
- Structure
- Functions
- Knockout mice
- Clinical significance
- Ligands
- References
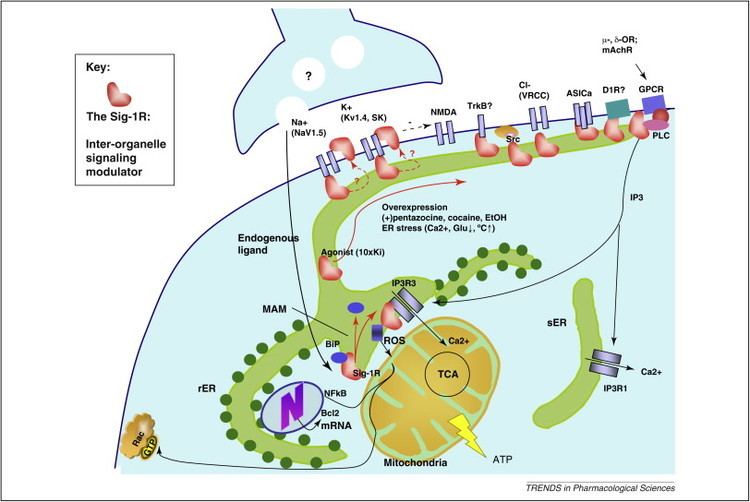
The σ1 receptor is a transmembrane protein expressed in many different tissue types. It is particularly concentrated in certain regions of the central nervous system. It has been implicated in several phenomena, including cardiovascular function, schizophrenia, clinical depression, the effects of cocaine abuse, and cancer. Much is known about the binding affinity of hundreds of synthetic compounds to the σ1 receptor.
An endogenous ligand for the σ1 receptor has yet to be conclusively identified, but tryptaminergic trace amines, as well as neuroactive steroids such as dehydroepiandrosterone (DHEA) and pregnenolone all activate the receptor.
Characteristics
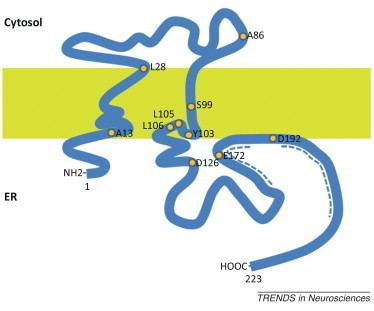
The σ1 receptor is defined by its unique pharmacological profile. In 1976 Martin reported that the effects of N-allylnormetazocine (SKF-10,047) could not be due to activity at the μ and κ receptors (named from the first letter of their selective ligands morphine and ketazocine, respectively) and a new type of opioid receptor was proposed; σ (from the first letter of SKF-10,047). However, ligands acting at this new “opioid” receptor were not blocked by the classical opioid antagonists naloxone and naltrexone. Consequently, the opioid classification was eventually dropped and the receptor was later termed the σ1 receptor. It was found to have affinity for the (+)-stereoisomers of several benzomorphans (e.g., (+)-pentazocine and (+)-cyclazocine), various structurally and pharmacologically distinct psychoactive chemicals such as haloperidol and cocaine, and neuroactive steroids like progesterone.
Structure
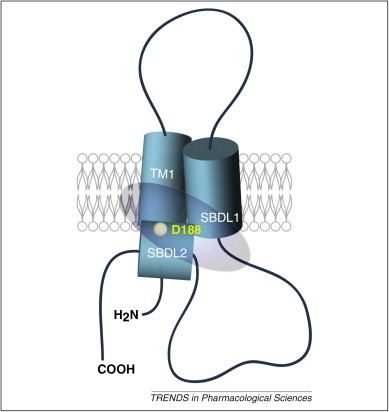
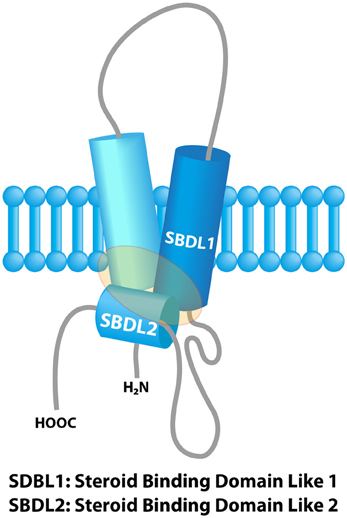
The mammalian σ1 receptor is an integral membrane protein with 223 amino acids. It shows no homology to other mammalian proteins but strikingly shares 30% sequence identity and 69% similarity with the ERG2 gene product of yeast, which is a C 8-C7 sterol isomerase in the ergosterol biosynthetic pathway. Hydropathy analysis of the σ1 receptor indicates three hydrophobic regions. A crystal structure of the σ1 receptor was published in 2016.
Functions
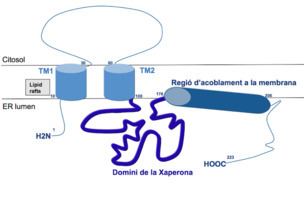
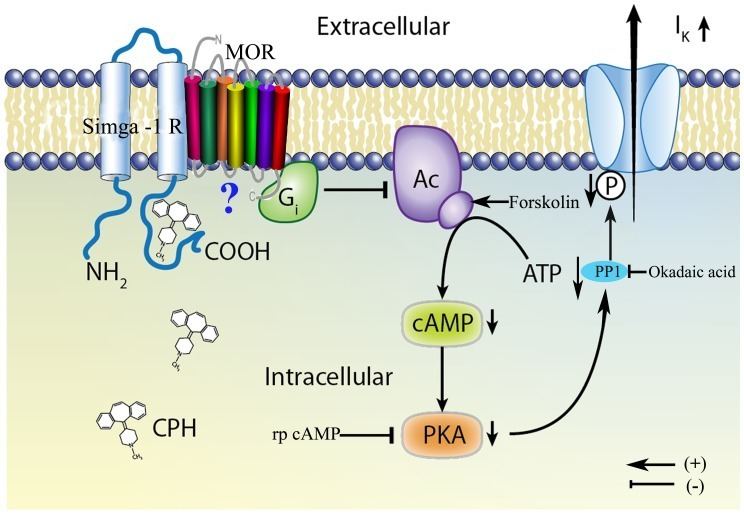
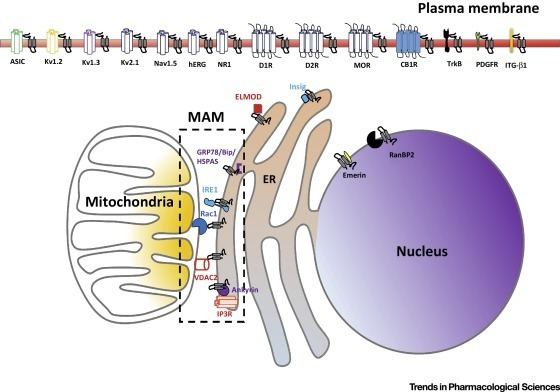
A variety of specific physiological functions have been attributed to the σ1 receptor. Chief among these are modulation of Ca2+ release, modulation of cardiac myocyte contractility, and inhibition of voltage gated K+ channels. The reasons for these effects are not well understood, even though σ1 receptors have been linked circumstantially to a wide variety of signal transduction pathways. Links between σ1 receptors and G-proteins have been suggested such as σ1 receptor antagonists showing GTP-sensitive high affinity binding, there is also, however, some evidence against a G-protein coupled hypothesis. The σ1 receptor has been shown to appear in a complex with voltage gated K+ channels (Kv1.4 and Kv1.5), leading to the idea that σ1 receptors are auxiliary subunits. σ1 receptors apparently co-localize with IP3 receptors on the endoplasmic reticulum. Also, σ1 receptors have been shown to appear in galactoceramide enriched domains at the endoplasmic reticulum of mature oligodendrocytes. The wide scope and effect of ligand binding on σ1 receptors has led some to believe that σ1 receptors are intracellular signal transduction amplifiers.
Knockout mice
σ1 receptor knockout mice were created in 2009 to study the effects of endogenous DMT. Strangely, the mice demonstrated no overt phenotype. As expected, however, they did lack locomotor response to the σ ligand (+)-SKF-10,047 and displayed reduced response to formalin induced pain. Speculation has focused on the ability of other receptors in the σ family (e.g., σ2, with similar binding properties) to compensate for the lack of σ1 receptor.
Clinical significance
Mutations in sigma-1 receptor have been associated with distal spinal muscular atrophy type 2.
Ligands
The following ligands have high affinity for the σ1 receptor and possess high binding selectivity over the subtype σ2:
Agents exist that have high σ1 affinity but either lack subtype selectivity or have high affinity at other binding sites, thus being more or less dirty/multifunctional, like haloperidol. Furthermore, there is a wide range of agents with an at least moderate σ1 involvement in their binding profile.
