3DMet B00125 Formula C2H7N Density 670 kg/m³ | Appearance Colorless gas Boiling point 7 °C Molar mass 45.08 g/mol | |
Related amines Related compounds | ||
Dimethylamine is an organic compound with the formula (CH3)2NH. This secondary amine is a colorless, flammable gas with an ammonia-like odor. Dimethylamine is commonly encountered commercially as a solution in water at concentrations up to around 40%. In 2005, an estimated 270,000 tons were produced industrially, but it is also found as a natural product.
Contents
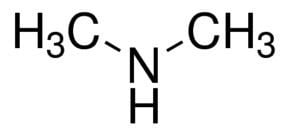
Structure and properties
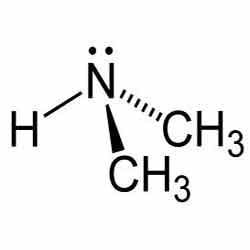
The molecule consists of a nitrogen atom with two methyl substituents and one proton. Dimethylamine is a weak base and the pKa of the ammonium salt CH3-NH2+-CH3 is 10.73, a value above methylamine (10.64) and trimethylamine (9.79).

Dimethylamine reacts with acids to form salts, such as dimethylamine hydrochloride, an odorless white solid with a melting point of 171.5 °C. Dimethylamine is produced by catalytic reaction of methanol and ammonia at elevated temperatures and high pressure:
2 CH3OH + NH3 → (CH3)2NH + 2 H2ONatural Occurrence
Dimethylamine is found quite widely distributed in animals and plants, and is present in many foods at the level of a few mg/kg.
Uses
Dimethylamine is a precursor to several industrially significant compounds. It reacts with carbon disulfide to give dimethyl dithiocarbamate, a precursor to a family of chemicals widely used in the vulcanization of rubber. The solvents dimethylformamide and dimethylacetamide are derived from dimethylamine. It is raw material for the production of many agrichemicals and pharmaceuticals, such as dimefox and diphenhydramine, respectively. The chemical weapon tabun is derived from dimethylamine. The surfactant lauryl dimethylamine oxide is found in soaps and cleaning compounds. Unsymmetrical dimethylhydrazine, a rocket fuel, is prepared from dimethylamine.
Biochemistry
The German cockroach utilizes dimethylamine as a pheromone for communication.
It is an attractant for boll weevils.
Dimethylamine undergoes nitrosation under weak acid conditions to give dimethylnitrosamine. This animal carcinogen has been detected and quantified in human urine samples and it may also arise from nitrosation of dimethylamine by nitrogen oxides present in acid rain in highly industrialized countries.
Toxicology
The toxicology of dimethylamine is discussed as part of a NIH monograph concerning dimethylamine borane. LD50: 736 mg/kg (mouse, i.p.); 316 mg/kg (mouse, p.o.); 698 mg/kg (rat, p.o.); 3900 mg/kg (rat, dermal); 240 mg/kg (guinea pig or rabbit, p.o.).
The unattributed LD (lethal dose) given in the Merck index for i.v. administration to rabbits, 4000 mg/kg, is unexpectedly high, and appears to be inconsistent with values given above.
