 | ||
Chondroitin sulfate is a sulfated glycosaminoglycan (GAG) composed of a chain of alternating sugars (N-acetylgalactosamine and glucuronic acid). It is usually found attached to proteins as part of a proteoglycan. A chondroitin chain can have over 100 individual sugars, each of which can be sulfated in variable positions and quantities. Chondroitin sulfate is an important structural component of cartilage and provides much of its resistance to compression. Along with glucosamine, chondroitin sulfate has become a widely used dietary supplement for treatment of osteoarthritis.
Contents
Medical use
Although chondroitin is used in dietary supplements as an alternative medicine to treat osteoarthritis and also approved and regulated as a symptomatic slow-acting drug for this disease (SYSADOA) in Europe and some other countries, it is technically neither a medicine nor a disease-modifying treatment. See Clinical effects below. It is commonly sold together with glucosamine. Chondroitin and glucosamine are also used in veterinary medicine. Formulated with collagen and wound dressing matrix, one product that uses chondroitin sulfate is the veterinary wound gel Chondroprotec, which is applied over scrapes, burns, and lesions and serves to keep the wound moist and promote healing.
Chondroitin, along with commonly used glucosamine, should not be used to treat patients who have symptomatic osteoarthritis of the knee as evidence shows that these treatments fail to provide relief for that condition.
Adverse effects
Clinical studies have not identified any significant side effects or overdoses of chondroitin sulfate, which suggest its long-term safety. The Task Force of the European League Against Rheumatism (EULAR) committee recently granted chondroitin sulfate a level of toxicity of 6 in a 0-100 scale, confirming it is one of the safest drugs for osteoarthritis. Moreover, its safety is supported by an absence of drug-drug interactions (chondroitin sulfate is not metabolized by cytochrome P450), and the lack of safe alternatives for patients multi-medicated for osteoarthritis and other accompanying diseases, e.g. diabetes, hypertension, hyperlipidemia, etc.
Physical and chemical properties
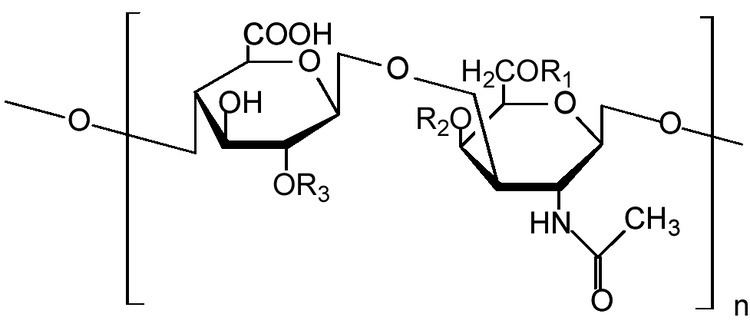
Chondroitin sulfate chains are unbranched polysaccharides of variable length containing two alternating monosaccharides: D-glucuronic acid (GlcA) and N-acetyl-D-galactosamine (GalNAc). Some GlcA residues are epimerized into L-iduronic acid (IdoA); the resulting disaccharide is then referred to as dermatan sulfate.
Protein attachment
Chondroitin sulfate chains are linked to hydroxyl groups on serine residues of certain proteins. Exactly how proteins are selected for attachment of glycosaminoglycans is not understood. Glycosylated serines are often followed by a glycine and have neighboring acidic residues, but this motif does not always predict glycosylation.
Attachment of the GAG chain begins with four monosaccharides in a fixed pattern: Xyl - Gal - Gal - GlcA. Each sugar is attached by a specific enzyme, allowing for multiple levels of control over GAG synthesis. Xylose begins to be attached to proteins in the endoplasmic reticulum, while the rest of the sugars are attached in the Golgi apparatus.
Interactions
Chondroitin's functions depend largely on the properties of the overall proteoglycan of which it is a part. These functions can be broadly divided into structural and regulatory roles. However, this division is not absolute, and some proteoglycans have both structural and regulatory roles (see versican).
Mechanisms of action
The effect of chondroitin sulfate in patients with osteoarthritis is likely the result of a number of reactions including its anti-inflammatory activity, the stimulation of the synthesis of proteoglycans and hyaluronic acid, and the decrease in catabolic activity of chondrocytes inhibiting the synthesis of proteolytic enzymes, nitric oxide, and other substances that contribute to damage cartilage matrix and cause death of articular chondrocytes. A recent review summarizes data from relevant reports describing the biochemical basis of the effect of chondroitin sulfate on osteoarthritis articular tissues. The rationale behind the use of chondroitin sulfate is based on the belief that osteoarthritis is associated with a local deficiency or degradation of natural substances, including internal chondroitin sulfate.
Recently, new mechanisms of action have been described for chondroitin sulfate. In an in vitro study, chondroitin sulfate reduced the IL-1β-induced nuclear factor-kB (NF-κB) translocation in chondrocytes. In addition, chondroitin sulfate has recently shown a positive effect on osteoarthritic structural changes occurred in the subchondral bone.
A review published in the Annals of Rheumatic Diseases describes all the documented evidence regarding the mechanisms of action of chondroitin sulfate
Bioavailability and pharmacokinetics
Pharmacokinetic studies performed on humans and experimental animals after oral administration of chondroitin sulfate revealed that it can be absorbed orally. Chondroitin sulfate shows first-order kinetics up to single doses of 3,000 mg. Multiple doses of 800 mg in patients with osteoarthritis do not alter the kinetics of chondroitin sulfate. The bioavailability of chondroitin sulfate ranges from 15% to 24% of the orally administered dose. More particularly, on the articular tissue, Ronca et al. reported that chondroitin sulfate is not rapidly absorbed in the gastro-intestinal tract and a high content of labeled chondroitin sulfate is found in the synovial fluid and cartilage.
Clinical effects
A 2015 Cochrane review of clinical trials found that most were of low quality, but that there was some evidence of short-term improvement in pain and few side effects; it does not appear to improve or maintain the health of affected joints.
The most relevant recently conducted clinical trials performed with chondroitin sulfate are summarized below.
The largest trial conducted with the product is the Glucosamine and Chondroitin Arthritis Intervention Trial (GAIT), a double-blind, randomized, multicenter clinical trial sponsored by the US National Institutes of Health in 1583 patients with knee osteoarthritis, which was published in the New England Journal of Medicine (Clegg DO, et al. 2006). Patients were randomly assigned to one of five orally administered treatments: two 250 mg capsules of glucosamine hydrochloride three times daily, two 200 mg capsules of chondroitin sulphate three times daily, two capsules of 250 mg of glucosamine hydrochloride plus 200 mg of chondroitin sulphate three times daily, 200 mg of celecoxib daily, or placebo. Treatment was administered for 24 weeks.
The primary outcome measure was a 20% decrease in the WOMAC pain subscale from baseline to week 24. The analysis of the primary outcome data for all patients showed the percentage of responders in each group to be: glucosamine: 64.0%; chondroitin sulphate: 65.4%; glucosamine + chondroitin sulphate: 66.6%; and celecoxib: 70.1% (p=0.008). Thus, despite the considerable effect elicited by all the products, only the celecoxib group reached statistical significance. This has been discussed by the authors and attributed mainly to the unprecedented high response in the placebo group (60.1%) and the relatively mild degree of pain among participants, which may have limited the ability to detect treatment benefits. Indeed, treatment effects were more substantial in patients with moderate-to-severe pain. Analysis of the results for the primary outcome, based on higher baseline pain, showed the percentage of responders in each treatment group to be 54.3% for placebo; 61.4% for chondroitin sulphate; 65.7% for glucosamine hydrochloride; 69.4% for celecoxib; and 79.2% for combined glucosamine and chondroitin sulphate. Thus, in the higher WOMAC pain stratum, only the combination glucosamine + chondroitin group presented significant efficacy (p=0.002).
Secondary efficacy outcome measures were as follows: OMERACT-OARSI response, 50% decrease in WOMAC pain score, WOMAC pain, stiffness and function score, normalized WOMAC score, patient and investigator global evaluations of disease status and response to study medication, evaluation of the index knee for swelling and tenderness, Health Assessment Questionnaire (HAQ) Alternative Disability score and HAQ Pain score, clinical evaluation for adverse reactions and reconciliation of study medications and rescue analgesia use. Analysis of the OMERACT-OARSI measure in all randomized patients revealed statistical significance for both celecoxib (p=0.007) and glucosamine and chondroitin in combination (P=0.02) compared to placebo. Swelling of study joints was significantly less in patients in the chondroitin sulphate (P=0.01) and celecoxib (P=0.03) treatment arms. Differences in the other secondary outcomes were not statistically significant when compared across the 5 treatment arms for all patients. For patients with moderate-to-severe pain at baseline (n=354), a statistically significant difference was observed in favor of the glucosamine and chondroitin combination group versus placebo for the following efficacy outcomes: 20% decrease in WOMAC pain score (0.002); OMERACT-OARSI response (0.001); 50% decrease in WOMAC pain score (0.02); WOMAC pain score (0.009); WOMAC function score (0.008); normalised WOMAC score (0.017); Health Assessment Questionnaire Pain score (0.03).
A subanalysis of GAIT results based on interaction of Kellgren & Lawrence Grade and Chondroitin Sulfate response relative to placebo was performed by the same study investigators. The results indicated better response for chondroitin sulfate relative to placebo for Kellgren & Lawrence grade 2 compared with grade 3. In general, chondroitin sulfate response within the Kellgren & Lawrence grade 2 group was very similar to that seen for celecoxib. These results suggest that chondroitin sulfate may improve osteoarthritis knee pain in patients with relatively early radiographic disease.
Sawitzke A, et al. 2010 evaluated the efficacy and safety of glucosamine and chondroitin sulfate, alone or in combination, as well as celecoxib and placebo on painful knee osteoarthritis over 2 years as a continuation of the GAIT trial. This was a 24-month, double-blind, placebo-controlled study, enrolling 662 patients with knee osteoarthritis who satisfied radiographic criteria (Kellgren/Lawrence grade 2 or 3 changes and baseline joint space width of at least 2 mm). This subset continued to receive their randomized treatment (glucosamine 500 mg three times daily, chondroitin sulfate 400 mg three times daily, the combination of glucosamine and chondroitin sulfate, celecoxib 200 mg daily, or placebo) over 24 months. The primary outcome was a 20% reduction in pain over 24 months as measured by the Western Ontario and McMaster University Osteoarthritis Index (WOMAC). Secondary outcomes included an Outcome Measures in Rheumatology/Osteoarthritis Research Society International response and change from baseline in WOMAC pain and function. Over 2 years, none of the treatments (not even the positive control celecoxib) achieved a clinically important difference in WOMAC pain or function as compared with placebo. Adverse reactions were similar among treatment groups and serious adverse events were rare for all treatments.
History
Chondroitin sulfate was originally isolated well before the structure was characterised, leading to changes in terminology with time. Early researchers identified different fractions of the substance with letters.
"Chondroitin sulfate B" is an old name for dermatan sulfate, and it is no longer classified as a form of chondroitin sulfate.
Chondroitin, without the "sulfate", has been used to describe a fraction with little or no sulfation. However, this distinction is not used by all.
Although the name "chondroitin sulfate" suggests a salt with a sulfate counter-anion, this is not the case, as sulfate is covalently bonded to the sugar. Rather, since the molecule has multiple negative charges at physiological pH, a cation is present in salts of chondroitin sulfate. Commercial preparations of chondroitin sulfate typically are the sodium salt. Barnhill et al. have suggested that all such preparations of chondroitin sulfate be referred to as "sodium chondroitin" regardless of their sulfation status.
Manufacture
Most chondroitin appears to be made from extracts of cartilaginous cow and pig tissues (cow trachea and pig ear and nose), but other sources such as shark, fish, and bird cartilage are also used. Since chondroitin is not a uniform substance, and is naturally present in a wide variety of forms, the precise composition of each supplement will vary. In fact, although many food supplement companies produce their products in compliance with human food processing Good Manufacturing Practice (GMP), most of them do not produce their products in compliance with the GMP regulations for pharmaceuticals, resulting in products without pharmaceutical requirements. Companies have attempted to produce chondroitin from other substances but have not yet had success.
Recent testing has revealed several flaws in the older testing methods. Without knowing the source of the chondroitin (e.g., shark, porcine, or bovine) and the approximate age of the animal, it is impossible to get a reliable reference standard, and, thus, results from previous testing had yielded percentages between 50 and 400%. In 2007, David Ji et al. reported in the Journal of Analytical Chemistry an extremely accurate method of quantification. The method included using an enzyme to break the chondroitin into its individual unsaturated disaccharides, and then measuring them using HPLC with an ion-pairing column and UV detection.
Legal status
While it is a prescription or over-the-counter drug in 22 countries, chondroitin is regulated in the U.S. as a dietary supplement by the Food and Drug Administration. In Europe, chondroitin sulfate formulations are approved as drugs with evidenced efficacy and safety demonstrated by clinical trials in osteoarthritic patients. Adebowale et al. reported in 2000 that of 32 chondroitin supplements they analysed, only 5 were labeled correctly, and more than half contained less than 40% of the labeled amount. With the introduction of GMP regulations for dietary supplements in 2008, chondroitin sulfate preparations are subject in the US to mandatory labeling standards as well as testing requirements for identity, purity, strength, and composition. United States Pharmacopoeia (USP) testing standards for the identification and quantification of chondroitin are well-established.
There are no FDA regulations on chondroitin sulfate as a food additive, as it is recognized by the FDA as a component of food and is "generally recognized as safe". However, a proposed application of chondroitin sulfate dietary supplement as a means of preventing joint degeneration was highly scrutinized by the FDA, who stated:
" For conventional foods, this evaluation involves considering whether the ingredient that is the source of the substance is generally recognized as safe (GRAS), approved as a food additive, or authorized by a prior sanction issued by FDA (see 21 CFR 101.70(f)). Dietary ingredients in dietary supplements, however, are not subject to the food additive provisions of the act (see section 201(s)(6) of the Act (21 U.S.C. § 321(s)(6)). Rather, they are subject to the adulteration provisions in section 402 of the Act (21 U.S.C. 342) and, if applicable, the new dietary ingredient provisions in section 413 of the Act (21 U.S.C. 350b), which pertain to dietary ingredients that were not marketed in the United States before October 15, 1994."
In the same letter, the FDA found that studies performed on the dietary supplement form of chondroitin sulfate were insufficient to substantiate claims that it is efficacious in the prevention of joint deterioration, and denied the request to be allowed to label the supplement as such. They further denied the request to market it as safe, given that no human clinical trials were done, citing that animal studies are not sufficient for the approval of a dietary supplement.
Clinical trials for osteoarthritis
In 2004, a petition was submitted to the FDA that a dietary supplement of chondroitin sulfate be labeled as reducing the risk of osteoarthritis, cartilage deterioration, and osteoarthritis-related joint pain, tenderness, and swelling. The FDA denied the request, stating that experiments conducted by the company did not sufficiently demonstrate the effectiveness of the claim. Among other comments, the FDA noted the poor experimental design of some trials.
In 2007, Reichenbach et al. used explicit methods to conduct and report a systematic review of 20 trials and concluded "large-scale, methodologically sound trials indicate that the symptomatic benefit of chondroitin is minimal or nonexistent. Use of chondroitin in routine clinical practice should therefore be discouraged." In contrast, and also in 2007, Bruyere et al. concluded that "there is compelling evidence that glucosamine sulfate and chondroitin sulfate may interfere with progression of OA."
Contamination in heparin
On Wednesday, March 19, 2008 the U.S. Food and Drug Administration (FDA) identified "oversulfated chondroitin sulfate" as a contaminant in heparin originating from China.
In this regard, "it is very important to remark on the relevant chemical differences between the chondroitin sulfate formulation approved in Europe as a drug and considered the reference product, and the "oversulfated chondroitin sulfate" identified as a contaminant in heparin originating from China."
The "oversulfated chondroitin sulfate" is not a product extracted from biological sources; it is synthesized through a sulfation reaction from the biological molecule. This is a semi-synthesis process that uses naturally derived chondroitin sulfate as a reagent in combination with various potentially dangerous chemicals, though their significance in the toxicity of "oversulfated chondroitin sulfate" is not known.
The resulting product contains 3 or 4 sulfate groups per disaccharide, and, therefore, its structure differs considerably from the original one (see #Sulfation section above). Furthermore, analysis of the contaminant unexpectedly revealed an unusual type of sulfation not found in any natural sources of chondroitin sulfate. In addition, a tetrasulfated disaccharide repeat unit has not been isolated to date from animal tissues
Thus, chondroitin sulfate is merely the substrate of the reaction: The final, oversulfated molecule constitutes a new entity, whose pharmacological and clinical properties are most likely very different from the biological molecule, as it is demonstrated in a recent article published in New England Journal of Medicine. In this study, Sasisekharan and colleagues showed that the oversulfated chondroitin sulfate (OSCS) found in contaminated lots of unfractionated heparin, as well as a synthetically generated OSCS reference standard, directly activated the kinin–kallikrein pathway in human plasma, which can lead to the generation of bradykinin, a potent vasoactive mediator. In addition, OSCS induced generation of C3a and C5a, potent anaphylatoxins derived from complement proteins. Chondroitin sulfate A was also tested and it showed no induction of amidollytic activity. Screening of plasma samples from various species indicated that swine and humans are sensitive to the effects of OSCS in a similar manner. OSCS-containing heparin and synthetically derived OSCS-induced hypotension associated with kallikrein activation when administered by intravenous infusion in swine. In contrast, none of the three pigs treated with chondroitin sulfate A showed any significant changes in blood pressure or heart rate.
