 | ||
In statistical mechanics and mathematics, a Boltzmann distribution (also called Gibbs distribution) is a probability distribution, probability measure, or frequency distribution of particles in a system over various possible states. The distribution is expressed in the form
Contents
where
In statistical mechanics, the Boltzmann distribution is a probability distribution that gives the probability that a system will be in a certain state as a function of that state’s energy and the temperature of the system. It is given as
where pi is the probability of state i, εi the energy of state i, k the Boltzmann constant, T the temperature of the system and M is the number of states accessible to the system. The sum is over all states accessible to the system of interest. The term system here has a very wide meaning; it can range from a single atom to a macroscopic system such as a natural gas storage tank. Because of this Boltzmann distribution can be used to solve a very wide variety of problems. The distribution shows that states with lower energy will always have a higher probability of being occupied than the states with higher energy.
The ratio of a Boltzmann distribution computed for two states is known as the Boltzmann factor and characteristically only depends on the states' energy difference.
The Boltzmann distribution is named after Ludwig Boltzmann who first formulated it in 1868 during his studies of the statistical mechanics of gases in thermal equilibrium. The distribution was later investigated extensively, in its modern generic form, by Josiah Willard Gibbs in 1902.
The Boltzmann distribution should not be confused with the Maxwell-Boltzmann distribution. The former gives the probability that a system will be in a certain state as a function of that state's energy. When applied to particles such as atoms or molecules, it gives the distribution of particles over energy states. The Maxwell-Boltzmann distribution is used to describe particle speeds in idealized gases.
The distribution
The Boltzmann distribution is a probability distribution that gives the probability of a certain state as a function of that state’s energy and temperature of the system to which the distribution is applied. It is given as
where pi is the probability of state i, εi the energy of state i, k the Boltzmann constant, T the temperature of the system and M is the number of all states accessible to the system. The sum is over all states accessible to the system of interest. The right hand side denominator of the equation above is also known as the canonical partition function, commonly denoted by Q (or by some authors by Z).
Therefore, the Boltzmann distribution can also be written as
The partition function can be calculated if we know the energies of the levels accessible to the system of interest. For atoms the partition function values can be found in the NIST Atomic Spectra Database.
The distribution shows that states with lower energy will always have a higher probability of being occupied than the states with higher energy. It can also give us the quantitative relationship between the probabilities of the two states being occupied. The ratio of probabilities for states i and j is given as
where pi is the probability of state i, pj the probability of state j, and εi and εi are the energies of states i and j, respectively.
The Boltzmann distribution is often used to describe the distribution of particles, such as atoms or molecules, over energy states accessible to them. If we have a system consisting of many particles, the probability of a particle being in state i is practically the probability that, if we pick a random particle from that system and check what state it is in, we will find it is in state i. This probability is equal to the number of particles in state i divided by the total number of particles in the system, that is the fraction of particles that occupy state i.
where Ni is the number of particles in state i and N is the total number of particles in the system. We may use the Boltzmann distribution to find this probability that is, as we have seen, equal to the fraction of particles that are in state i. So the equation that gives the fraction of particles in state i as a function of the energy of that state is
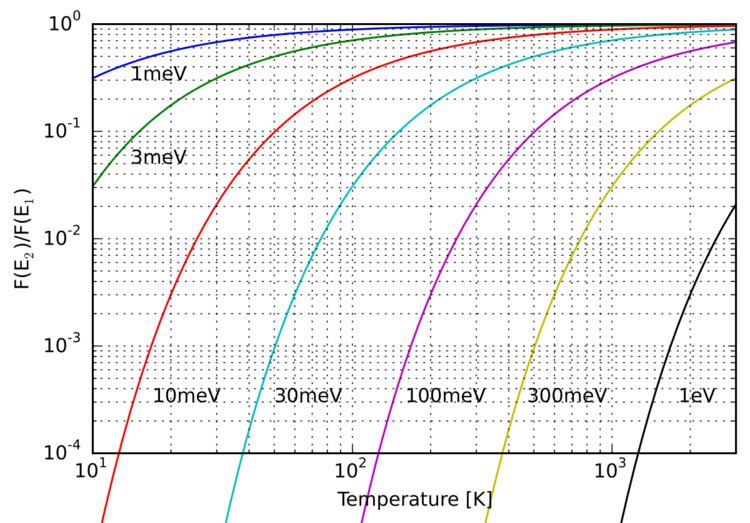
This equation is of great importance to spectroscopy. In spectroscopy we observe a spectral line if atoms or molecules that we are interested in going from one state to another. In order for this to be possible, there must be some particles in the first state to undergo the transition. We may find that this condition is fulfilled by finding the fraction of particles in the first state. If it is negligible, the transition is very likely not be observed at the temperature for which the calculation was done. In general, a larger fraction of molecules in the first state means a higher number of transitions to the second state. This gives a stronger spectral line. However, there are other factors that influence the intensity of a spectral line, such as whether it is caused by an allowed or a forbidden transition.
In statistical mechanics
The Boltzmann distribution appears in statistical mechanics when considering isolated (or nearly-isolated) systems of fixed composition that are in thermal equilibrium (equilibrium with respect to energy exchange). The most general case is the probability distribution for the canonical ensemble, but also some special cases (derivable from the canonical ensemble) also show the Boltzmann distribution in different aspects:
Although these cases have strong similarities, it is helpful to distinguish them as they generalize in different ways when the crucial assumptions are changed:
In mathematics
In more general mathematical settings, the Boltzmann distribution is also known as the Gibbs measure. In statistics and machine learning it is called a log-linear model.
In economics
The Boltzmann distribution can be introduced to allocate permits in emissions trading. The new allocation method using the Boltzmann distribution can describe the most probable, natural, and unbiased distribution of emissions permits among multiple countries. Simple and versatile, this new method holds potential for many economic and environmental applications.
