 | ||
A biosafety level is a set of biocontainment precautions required to isolate dangerous biological agents in an enclosed laboratory facility. The levels of containment range from the lowest biosafety level 1 (BSL-1) to the highest at level 4 (BSL-4). In the United States, the Centers for Disease Control and Prevention (CDC) have specified these levels. In the European Union, the same biosafety levels are defined in a directive. Facilities with these designations are also sometimes given as P1 through P4 (for Pathogen or Protection level), as in the term "P3 laboratory".
Contents
- History
- Biosafety level 1
- Biosafety level 2
- Biosafety level 3
- Biosafety level 4
- List of BSL 4 facilities
- Safety concerns
- References
At the lowest level of biosafety, precautions may consist of regular hand-washing and minimal protective equipment. At higher biosafety levels, precautions may include airflow systems, multiple containment rooms, sealed containers, positive pressure personnel suits, established protocols for all procedures, extensive personnel training, and high levels of security to control access to the facility.
History
The first prototype Class III (maximum containment) biosafety cabinet was fashioned in 1943 by Hubert Kaempf Jr., then a U.S. Army soldier, under the direction of Dr. Arnold G. Wedum, Director (1944–69) of Industrial Health and Safety at the United States Army Biological Warfare Laboratories, Camp Detrick, Maryland. Kaempf was tired of his MP duties at Detrick and was able to transfer to the sheet metal department working with the contractor, the H.K. Ferguson Co.
On 18 April 1955, fourteen representatives met at Camp Detrick in Frederick, Maryland. The meeting was to share knowledge and experiences regarding biosafety, chemical, radiological, and industrial safety issues that were common to the operations at the three principal biological warfare (BW) laboratories of the U.S. Army. Because of the potential implication of the work conducted at biological warfare laboratories, the conferences were restricted to top level security clearances. Beginning in 1957, these conferences were planned to include non-classified sessions as well as classified sessions to enable broader sharing of biological safety information. It was not until 1964, however, that conferences were held in a government installation not associated with a biological warfare program.
Over the next ten years, the biological safety conferences grew to include representatives from all federal agencies that sponsored or conducted research with pathogenic microorganisms. By 1966 it began to include representatives from universities, private laboratories, hospitals, and industrial complexes. Throughout the 1970s, participation in the conferences continued to expand and by 1983 discussions began regarding the creation of a formal organization. The American Biological Safety Association (ABSA) was officially established in 1984 and a constitution and bylaws were drafted the same year. As of 2008, ABSA includes some 1,600 members in its professional association.
Biosafety level 1
Biosafety level 1 (BSL-1) is suitable for work with well-characterized agents which do not cause disease in healthy humans. In general, these agents should pose minimal potential hazard to laboratory personnel and the environment. At this level, precautions are limited relative to other levels. Laboratory personnel must wash their hands upon entering and exiting the lab. Research with these agents may be performed on standard open laboratory benches without the use of special containment equipment. However, eating and drinking are generally prohibited in laboratory areas. Potentially infectious material must be decontaminated before disposal, either by adding an appropriate disinfectant, or by packaging for decontamination elsewhere. Personal protective equipment is only required for circumstances where personnel might be exposed to hazardous material. BSL-1 laboratories must have a door which can be locked to limit access to the lab, however it is not necessary for BSL-1 labs to be isolated from the general building.
This level of biosafety is appropriate for work with several kinds of microorganisms including non-pathogenic Escherichia coli, Bacillus subtilis, Saccharomyces cerevisiae and other organisms not suspected to contribute to human disease. Due to the relative ease and safety of maintaining a BSL-1 laboratory, these are the types of laboratories generally used as teaching spaces for high schools and colleges.
Biosafety level 2
At this level, all precautions used at Biosafety Level 1 are followed, and some additional precautions are taken. BSL-2 differs from BSL-1 in that:
Biosafety level 2 is suitable for work involving agents of moderate potential hazard to personnel and the environment. This includes various microbes that cause mild disease to humans, or are difficult to contract via aerosol in a lab setting. Examples include Hepatitis A, B, and C viruses, human immunodeficiency virus (HIV), pathogenic Escherichia coli, Staphylococcus aureus, Salmonella, Plasmodium falciparum, and Toxoplasma gondii.
Biosafety level 3
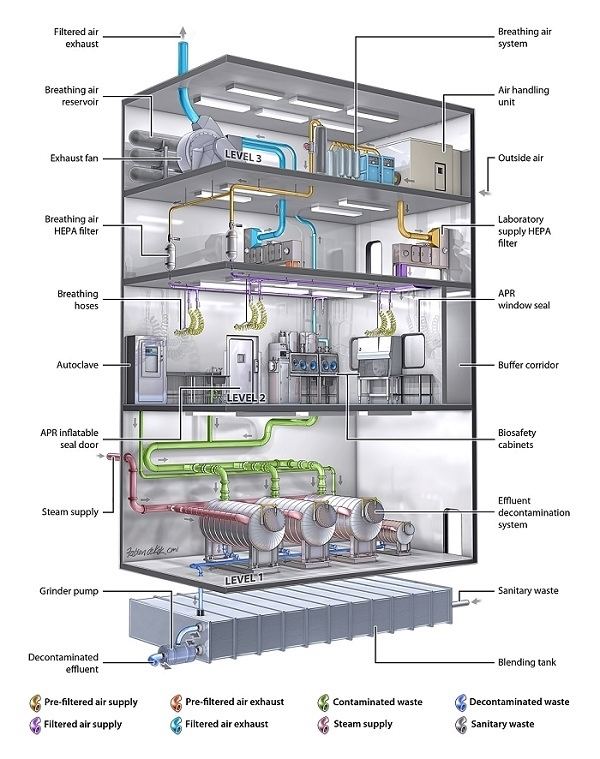
Biosafety level 3 is appropriate for work involving microbes which can cause serious and potentially lethal disease via the inhalation route. This type of work can be done in clinical, diagnostic, teaching, research, or production facilities. Here, the precautions undertaken in BSL-1 and BSL-2 labs are followed, as well as additional measures including:
In addition, the facility which houses the BSL-3 laboratory must have certain features to ensure appropriate containment. The entrance to the laboratory must be separated from areas of the building with unrestricted traffic flow. Additionally, the laboratory must be behind two sets of self-closing doors (to reduce the risk of aerosols escaping). The construction of the laboratory is such that it can be easily cleaned. Carpets are not permitted, and any seams in the floors, walls, and ceilings are sealed to allow for easy cleaning and decontamination. Additionally, windows must be sealed, and a ventilation system installed which forces air to flow from the "clean" areas of the lab to the areas where infectious agents are handled. Air from the laboratory must be filtered before it can be recirculated.
Biosafety level 3 is commonly used for research and diagnostic work involving various microbes which can be transmitted by aerosols and/or cause severe disease. These include Francisella tularensis, Mycobacterium tuberculosis, Chlamydia psittaci, Venezuelan equine encephalitis virus, Eastern equine encephalitis virus, SARS coronavirus, Coxiella burnetii, Rift Valley fever virus, Rickettsia rickettsii, several species of Brucella, chikungunya, yellow fever virus, and West Nile virus.
Biosafety level 4
Biosafety level 4 (BSL-4) is the highest level of biosafety precautions, and is appropriate for work with agents that could easily be aerosol-transmitted within the laboratory and cause severe to fatal disease in humans for which there are no available vaccines or treatments. BSL-4 laboratories are generally set up to be either cabinet laboratories or protective suit laboratories. In cabinet laboratories, all work must be done within a class III biosafety cabinet. Materials leaving the cabinet must be decontaminated by passing through an autoclave or a tank of disinfectant. The cabinets themselves are required to have seamless edges to allow for easy cleaning. Additionally the cabinet and all materials within must be free of sharp edges in order to reduce the risk of damage to the gloves. In a protective suit laboratory, all work must be done in a class II biosafety cabinet by personnel wearing a positive pressure suit. In order to exit the BSL-4 laboratory, personnel must pass through a chemical shower for decontamination, then a room for removing the positive pressure suit, followed by a personal shower. Entry into the BSL-4 laboratory is restricted to trained and authorized individuals, and all persons entering and exiting the laboratory must be recorded.
As with BSL-3 laboratories, BSL-4 laboratories must be separated from areas that receive unrestricted traffic. Additionally airflow is tightly controlled to ensure that air always flows from "clean" areas of the lab to areas where work with infectious agents is being performed. The entrance to the BSL-4 lab must also employ airlocks to minimize the possibility that aerosols from the lab could be removed from the lab. All laboratory waste, including filtered air, water, and trash must also be decontaminated before it can leave the facility.
Biosafety level 4 laboratories are used for diagnostic work and research on easily transmitted pathogens which can cause fatal disease. These include a number of viruses known to cause viral hemorrhagic fever such as Marburg virus, Ebola virus, Lassa virus, Crimean-Congo hemorrhagic fever. Other pathogens handled at BSL-4 include Hendra virus, Nipah virus, and some Flaviviruses. Additionally, poorly characterized pathogens which appear closely related to dangerous pathogens are often handled at this level until sufficient data are obtained either to confirm continued work at this level, or to work with them at a lower level. This level is also used for work with Variola virus, the causative agent of smallpox, though this work can only be done at the World Health Organization-approved facilities at the Centers for Disease Control and Prevention in Atlanta, U.S.A. as well as the State Research Center of Virology and Biotechnology in Koltsovo, Russia.
List of BSL-4 facilities
According to the U.S. Government Accountability Office (GAO) report published on October 4, 2007, a total of 1,356 CDC/USDA registered BSL-3 facilities were identified throughout the United States. Approximately 36% of these laboratories are located in academia. 15 BSL-4 facilities were identified in the U.S. in 2007, including nine at federal labs.
The following is a list of existing BSL-4 facilities worldwide.
Safety concerns
A North Carolina Mosquito & Vector Control Association (NCMVCA) study highlighted safety concerns. In the United States laboratories can be funded by federal, state, private, non-profit, or academically. The last accounts for 72% of the funding. There is no central monitoring agency accountable for monitoring laboratories and standards vary according to funding, the age of the laboratory, and is dependent on the size and if it is SA approved or not.
High-containment labs that are registered with the Centers for Disease Control and Prevention (CDC) and the U.S. Department of Agriculture's (USDA) Select Agent Program must adhere to Department of Defense standards. No single federal agency, according to 12 agencies' responses to a GSA survey, has the mission to track the overall number of BSL-3 and BSL-4 labs in the United States. This means no agency is responsible for determining the risks associated with the proliferation of these labs.
