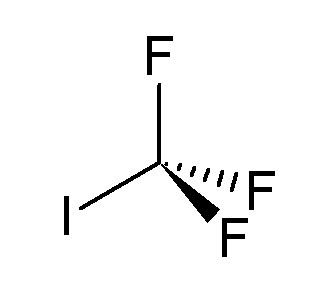
Appearance Colorless odorless gas Molar mass 195.91 g/mol EU classification (DSD) Muta. Cat. 3 Spectral data UV, IR, NMR, MS | Formula CF3I Density 2.55 g/cm³ R-phrases R68 | |
 | ||
Thermodynamic data Phase behaviour; solid–liquid–gas | ||
Trifluoroiodomethane, also referred to as trifluoromethyl iodide is a halomethane with the formula CF3I. It is an experimental alternative to Halon 1301 (CBrF3) in unoccupied areas. It would be used as a gaseous fire suppression flooding agent for in-flight aircraft and electronic equipment fires.
Contents
Chemistry
It is used in the rhodium-catalyzed α-trifluoromethylation of α,β-unsaturated ketones.
In the presence of sunlight or at temperatures above 100 °C it can react with water, forming hazardous by-products such as hydrogen fluoride (HF), hydrogen iodide (HI) and carbonyl fluoride (COF2).
Environmental effects
Trifluoroiodomethane contains carbon, fluorine, and iodine atoms. Although iodine is several hundred times more efficient at destroying stratospheric ozone than chlorine, experiments have shown that because the weak C-I bond breaks easily under the influence of water (owing to the electron-attracting fluorine atoms), trifluoroiodomethane has an ozone depleting potential less than one-thousandth that of Halon 1301 (0.008-0.01). Its atmospheric lifetime, at less than 1 month, is less than 1 percent that of Halon 1301, and less even than hydrogen chloride formed from volcanoes.
There is, however, still the problem of the C-F bonds absorbing in the atmospheric window. However, the IPCC has calculated the 100-year global warming potential of trifluoroiodomethane to be 0.4 (i.e., 40% of that of CO2).
