Appearance Colorless gas Molar mass 66.01 g/mol Density 2.7 g/cm³ | Formula COF2 Boiling point -84.57 °C | |
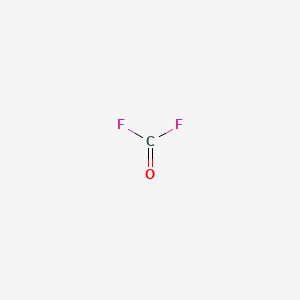 | ||
Related compounds | ||
Carbonyl fluoride is the inorganic compound with the formula COF2. This gas, like its analog phosgene, is colourless and highly toxic. The molecule is planar with C2v symmetry.
Contents
Preparation and properties
Carbonyl fluoride can be prepared by reaction of phosgene with hydrogen fluoride and the oxidation of carbon monoxide, although the latter tends to result in over-oxidation to carbon tetrafluoride. The oxidation of carbon monoxide with silver difluoride is convenient:
CO + 2 AgF2 → COF2 + 2 AgFCarbonyl fluoride is unstable in the presence of water, hydrolyzing to carbon dioxide and hydrogen fluoride:
COF2 + H2O → CO2 + 2 HFSafety
Carbonyl fluoride is extremely poisonous with a threshold limit value of 2 ppm for short-term exposure.
References
Carbonyl fluoride Wikipedia(Text) CC BY-SA
