eMedicine med/2286 emerg/596 MeSH D016171 | DiseasesDB 29252 Patient UK Torsades de pointes | |
 | ||
Torsades de pointes or torsade de pointes (TdP or simply torsade(s)) ([tɔʁsad də pwɛ̃t], translated as "twisting of the points"), is a specific type of abnormal heart rhythm that can lead to sudden cardiac death. It is a polymorphic ventricular tachycardia that exhibits distinct characteristics on the electrocardiogram (ECG). It was described by Dessertenne in 1966. Prolongation of the QT interval can increase a person's risk of developing this abnormal heart rhythm.
Contents
Pathophysiology
Action potential of cardiac muscles can be broken down into five phases:
Repolarization of the cardiomyocytes occurs in phases 1-3, and is caused predominantly by the outward movement of potassium ions. In Torsades de pointes, however, the repolarization is prolonged; this can be due to electrolyte disturbances (hypokalemia, hypomagnesemia, hypocalcemia), bradycardia, certain drugs (disopyramide, sotalol, amiodarone, amitriptyline, chlorpromazine, erythromycin) and/or congenital syndromes (LQT1-LQT4 gene defect).
The prolongation of repolarisation may result in subsequent activation of an inward depolarisation current, known as an early after-depolarisation, which may promote triggered activity. Re-entry, due to a dispersion of refractory periods, is also possible; this is because M Cells (found in the mid myocardial layer) show a more prolonged repolarization phase in response to potassium blockage than other cells. In turn, this produces a zone of functional refractoriness (inability to depolarize) in the mid myocardial layer. When new action potential is generated, the mid myocardial layer will remain in a refractory period, but the surrounding tissue will depolarize. As soon as the mid myocardial layer is no longer in a refractory period, excitation from nearby tissue will cause a retrograde current and a reentry circuit that will result in a positive chronotropic cycle, leading to tachycardia.
Signs and symptoms
Most episodes will revert spontaneously to a normal sinus rhythm. If this does not occur, however, possible adverse outcomes include palpitations, dizziness, lightheadedness (during shorter episodes), fainting (during longer episodes), and sudden cardiac death.
Causes
Common causes for torsades de pointes include diarrhea, low blood magnesium, and low blood potassium. It is commonly seen in malnourished individuals and chronic alcoholics, since they are often deficient in potassium and/or magnesium. Certain combinations of drugs resulting in drug interactions can contribute to torsades de pointes risk. QT prolonging medications such as clarithromycin, levofloxacin, or haloperidol, when taken concurrently with a specific cytochrome P450 inhibitor, such as fluoxetine, cimetidine, or certain foods like grapefruit, can result in higher-than-normal levels of QT prolonging medications in the bloodstream and therefore increase a person's risk of developing torsades de pointes. In addition, inherited long QT syndrome significantly increases the risk of episodes of TdP.
Medications
Knowledge that TdP may occur in patients taking certain prescription drugs has been both a major liability and reason for retirement of these medications from the marketplace. Examples of compounds linked to clinical observations of TdP include amiodarone, fluoroquinolones, methadone, lithium, chloroquine, erythromycin, amphetamine, ephedrine, pseudoephedrine, methylphenidate, and phenothiazines. It has also been shown as a side effect of certain anti-arrhythmic medications, such as sotalol, procainamide, and quinidine. The gastrokinetic drug cisapride (Propulsid) was withdrawn from the US market in 2000 after it was linked to deaths caused by long QT syndrome-induced torsades de pointes. In many cases, this effect can be directly linked to QT prolongation mediated predominantly by inhibition of the hERG channel.
In September 2011 (subsequently updated in March 2012 and February 2013), the FDA issued a warning concerning increased incidence of QT prolongation in patients prescribed doses of the antidepressant Celexa (citalopram) above 40 mg per day, considered the maximum allowable dosage, thereby increasing the risk of Torsades. However, a study, "Evaluation of the FDA Warning Against Prescribing Citalopram at Doses Exceeding 40 mg," reported no increased risk of abnormal arrhythmias, thus questioning the validity of the FDA's warning.
Risk factors
The following is a list of factors associated with an increased tendency towards developing torsades de pointes:
Diagnosis
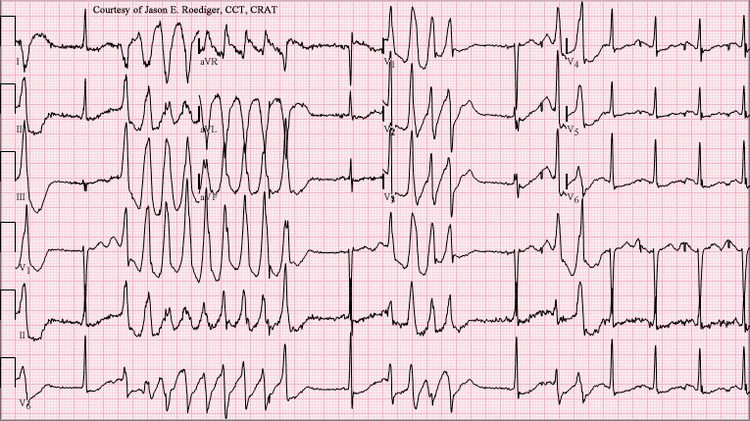
The ECG tracing in torsades demonstrates a polymorphic ventricular tachycardia with a characteristic illusion of a twisting of the QRS complex around the isoelectric baseline (peaks, which are at first pointing up, appear to be pointing down for subsequent "beats" when looking at ECG traces of the "heartbeat"). It is hemodynamically unstable and causes a sudden drop in arterial blood pressure, leading to dizziness and fainting. Depending on their cause, most individual episodes of torsades de pointes revert to normal sinus rhythm within a few seconds; however, episodes may also persist and possibly degenerate into ventricular fibrillation, leading to sudden death in the absence of prompt medical intervention. Torsades de pointes is associated with long QT syndrome, a condition whereby prolonged QT intervals are visible on an ECG. Long QT intervals predispose the patient to an R-on-T phenomenon, wherein the R-wave, representing ventricular depolarization, occurs during the relative refractory period at the end of repolarization (represented by the latter half of the T-wave). An R-on-T can initiate torsades. Sometimes, pathologic T-U waves may be seen in the ECG before the initiation of torsades.
A "short-coupled variant of torsade de pointes", which presents without long QT syndrome, was also described in 1994 as having the following characteristics:
Treatment
Treatment is directed towards the withdrawal of the offending agent, infusion of magnesium sulfate, antiarrhythmic drugs, and electrical therapy, such as a temporary pacemaker, as needed.
Because of the polymorphic nature of torsades de pointes, synchronized cardioversion may not be possible, and the patient may require an unsynchronized shock (or defibrillation).
History
The phenomenon was originally described in a French medical journal by Dessertenne in 1966, when he observed this cardiac rhythm disorder in an 80-year-old female patient with complete intermittent atrioventricular block. In coining the term, he referred his colleagues to the "Dictionnaire Le Robert," a bilingual French English dictionary, of which his wife had just given him a copy. Here, "torsade" is defined as:
Terminology
The singular and plural forms (torsade de pointes and torsades de pointes) have both often been used. The question of whether either one is grammatically "correct" and the other "incorrect" has repeatedly arisen. This is seen among major medical dictionaries, where one may enter only the plural form, while another enters the plural form as the headword but lists the singular as a variant, and yet another enters the singular form as the headword, giving a usage comment saying that the plural is not preferred. One group of physicians has suggested that it would make the most sense to use the singular form to refer to the arrythmia entity (where an arrythmia may involve one or multiple episodes), and that one might best reserve the plural form for describing repeated twistings during a single episode. Regarding the natural language variation, they concluded, in good-nature: "Wasn't it the French who coined the term 'vive la difference?'"
