Related compounds Molar mass 84.14 g/mol Density 1.05 g/cm³ | Formula C4H4S Boiling point 84 °C Appearance colorless liquid | |
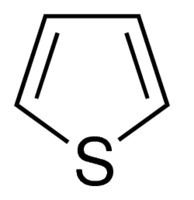 | ||
Thiophene, also commonly called thiofuran, is a heterocyclic compound with the formula C4H4S. Consisting of a flat five-membered ring, it is aromatic as indicated by its extensive substitution reactions. Related to thiophene are benzothiophene and dibenzothiophene, containing the thiophene ring fused with one and two benzene rings, respectively. Compounds analogous to thiophene include furan (C4H4O) and pyrrole (C4H4NH), which each vary by the heteroatom in the ring.
Contents
- Isolation and occurrence
- Synthesis and production
- Properties
- Reactivity
- Toward electrophiles
- Desulfurization by Raney nickel
- Coordination chemistry
- Thienyl
- Uses
- Polythiophene
- References
Isolation and occurrence
Thiophene was discovered as a contaminant in benzene. It was observed that isatin (an indole) forms a blue dye if it is mixed with sulfuric acid and crude benzene. The formation of the blue indophenin had long been believed to be a reaction of benzene itself. Viktor Meyer was able to isolate thiophene as the actual substance responsible for this reaction.

Thiophene and its derivatives occur in petroleum, sometimes in concentrations up to 1–3%. The thiophenic content of oil and coal is removed via the hydrodesulfurization (HDS) process. In HDS, the liquid or gaseous feed is passed over a form of molybdenum disulfide catalyst under a pressure of H2. Thiophenes undergo hydrogenolysis to form hydrocarbons and hydrogen sulfide. Thus, thiophene itself is converted to butane and H2S. More prevalent and more problematic in petroleum are benzothiophene and dibenzothiophene.
Synthesis and production
Reflecting their high stabilities, thiophenes arise from many reactions involving sulfur sources and hydrocarbons, especially unsaturated ones, e.g. acetylenes and elemental sulfur, which was the first synthesis of thiophene by Meyer the same year that he reported its discovery. Thiophenes are classically prepared by the reaction of 1,4-diketones, diesters, or dicarboxylates with sulfidizing reagents such as P4S10 such as in the Paal-Knorr thiophene synthesis. Specialized thiophenes can be synthesized similarly using Lawesson's reagent as the sulfidizing agent, or via the Gewald reaction, which involves the condensation of two esters in the presence of elemental sulfur. Another method is the Volhard–Erdmann cyclization.
Thiophene is produced on a scale of around 2,000,000 kilograms per year worldwide. Production involves the vapor phase reaction of a sulfur source, typically carbon disulfide, and butanol. These reagents are contacted with an oxide catalyst at 500–550 °C.
Properties
At room temperature, thiophene is a colorless liquid with a mildly pleasant odor reminiscent of benzene, with which thiophene shares some similarities. The high reactivity of thiophene toward sulfonation is the basis for the separation of thiophene from benzene, which are difficult to separate by distillation due to their similar boiling points (4 °C difference at ambient pressure). Like benzene, thiophene forms an azeotrope with ethanol.
The molecule is flat; the bond angle at the sulphur is around 93°, the C–C–S angle is around 109°, and the other two carbons have a bond angle around 114°. The C–C bonds to the carbons adjacent to the sulphur are about 1.34 Å, the C–S bond length is around 1.70 Å, and the other C–C bond is about 1.41 Å (figures from the Cambridge Structural Database).
Reactivity
Thiophene is considered to be aromatic, although theoretical calculations suggest that the degree of aromaticity is less than that of benzene. The "electron pairs" on sulfur are significantly delocalized in the pi electron system. As a consequence of its aromaticity, thiophene does not exhibit the properties seen for conventional thioethers. For example, the sulfur atom resists alkylation and oxidation. However, oxidation of a thiophene ring is thought to play a crucial role in the metabolic activation of various thiophene-containing drugs, such as tienilic acid and the investigational anticancer drug OSI-930. In these cases oxidation can occur both at sulfur, giving a thiophene S-oxide, as well as at the 2,3-double bond, giving the thiophene 2,3-epoxide, followed by subsequent NIH shift rearrangement. Oxidation of thiophene by trifluoroperacetic acid also demonstrates both reaction pathways. The major pathway forms the S-oxide as an intermediate, which undergoes subsequent Diels-Alder-type dimerisation and further oxidation, forming a mixture of sulfoxide and sulfone products with a combined yield of 83% (based on NMR evidence):
In the minor reaction pathway, a Prilezhaev epoxidation results in the formation of thiophene-2,3-epoxide that rapidly rearranges to the isomer thiophene-2-one. Trapping experiments demonstrate that this pathway is not a side reaction from the S-oxide intermediate, while isotopic labeling with deuterium confirm that a 1,2-hydride shift occurs and thus that a cationic intermediate is involved. Trifluoroperacetic acid is prepared immediately prior to use, and the method chosen determines whether or not water is present; if the reaction mixture is not anhydrous, this minor reaction pathway is suppressed as water acts as a competing base.
Toward electrophiles
Although the sulfur atom is relatively unreactive, the flanking carbon centers, the 2- and 5-positions, are highly susceptible to attack by electrophiles. Halogens give initially 2-halo derivatives followed by 2,5-dihalothiophenes; perhalogenation is easily accomplished to give C4X4S (X = Cl, Br, I). Thiophene brominates 107 times faster than does benzene.
Chloromethylation and chloroethylation occur readily at the 2,5-positions. Reduction of the chloromethyl product gives 2-methylthiophene. Hydrolysis followed by dehydration of the chloroethyl species gives 2-vinylthiophene.
Desulfurization by Raney nickel
Desulfurization of thiophene with Raney nickel affords butane. When coupled with the easy 2,5-difunctionalization of thiophene, desulfurization provides a route to 1,4-disubstituted butanes.
Coordination chemistry
Thiophene exhibits little thioether-like character, but it does serve as a pi-ligand forming piano stool complexes such as Cr(η5-C4H4S)(CO)3.
Thienyl
Upon deprotonation, thiophene converts to the thienyl group, C4H3S−. Although the anion per se do not exist, the organolithium derivatives do. Thus reaction of thiophene with butyl lithium gives 2-lithiothiophene, also called 2-thienyllithium. This reagent reacts with electrophiles to give thienyl derivatives, such as the thiol. Oxidation of thienyllithium gives 2,2'-dithienyl, (C4H3)2. Thienyl lithium is employed in the preparation of higher order mixed cuprates.
Uses
Thiophenes are important heterocyclic compounds that are widely used as building blocks in many agrochemicals and pharmaceuticals. The benzene ring of a biologically active compound may often be replaced by a thiophene without loss of activity. This is seen in examples such as the NSAID lornoxicam, the thiophene analog of piroxicam.
Polythiophene
The polymer formed by linking thiophene through its 2,5 positions is called polythiophene. Polythiophene itself has poor processing properties. More useful are polymers derived from thiophenes substituted at the 3- and 3- and 4- positions. Polythiophenes become electrically conductive upon partial oxidation, i.e. they obtain some of the characteristics typically observed in metals.
