Entrez 7350 | Ensembl ENSG00000109424 | |
 | ||
External IDs | ||
Uncoupling protein thermogenin heat production etc poisons
Thermogenin (called uncoupling protein by its discoverers and now known as uncoupling protein 1, or UCP1) is an uncoupling protein found in the mitochondria of brown adipose tissue (BAT). It is used to generate heat by non-shivering thermogenesis, and makes a quantitatively important contribution to countering heat loss in neonates which would otherwise occur due to the high surface area-volume ratio.
Contents
- Uncoupling protein thermogenin heat production etc poisons
- Thermogenin and proton transport uncouplers
- Mechanism
- History
- Clinical Relevance
- References
Thermogenin and proton transport uncouplers
Mechanism
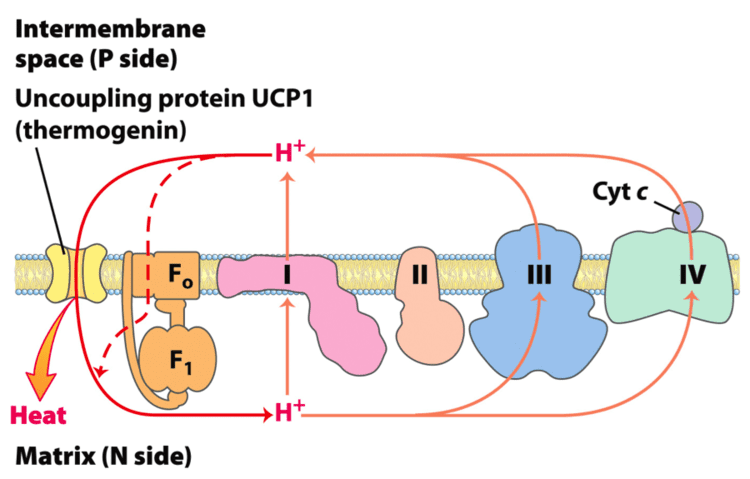
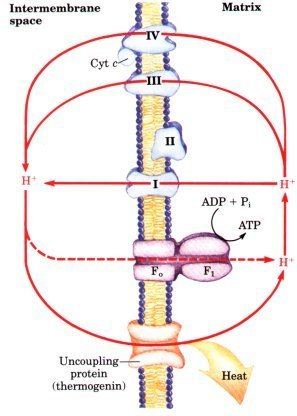
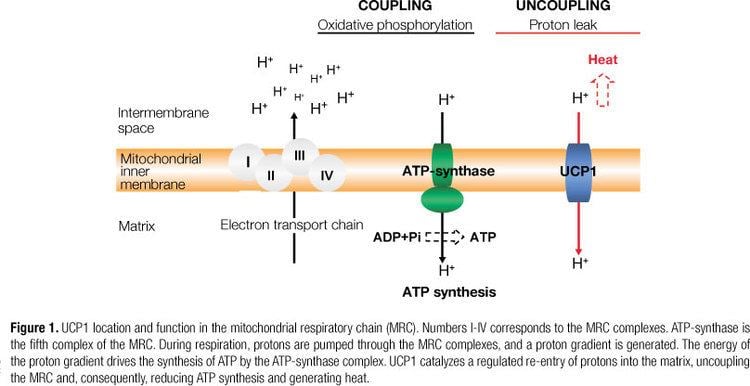
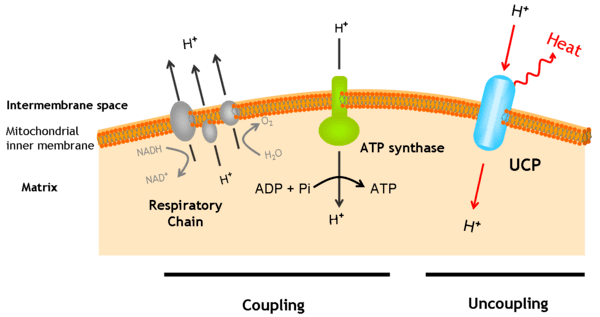
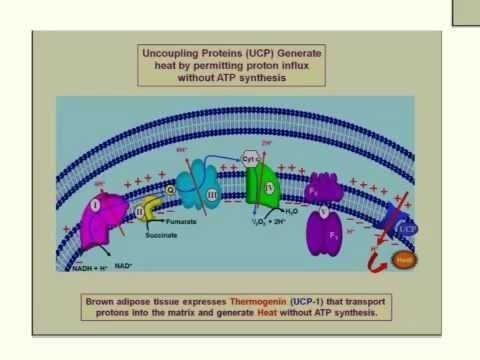
UCPs are transmembrane proteins that decrease the proton gradient generated in oxidative phosphorylation. They do this by increasing the permeability of the inner mitochondrial membrane, allowing protons that have been pumped into the intermembrane space to return to the mitochondrial matrix. UCP1-mediated heat generation in brown fat uncouples the respiratory chain, allowing for fast substrate oxidation with a low rate of ATP production. UCP1 is related to other mitochondrial metabolite transporters such as the adenine nucleotide translocator, a proton channel in the mitochondrial inner membrane that permits the translocation of protons from the mitochondrial intermembrane space to the mitochondrial matrix. UCP1 is restricted to brown adipose tissue, where it provides a mechanism for the enormous heat-generating capacity of the tissue.
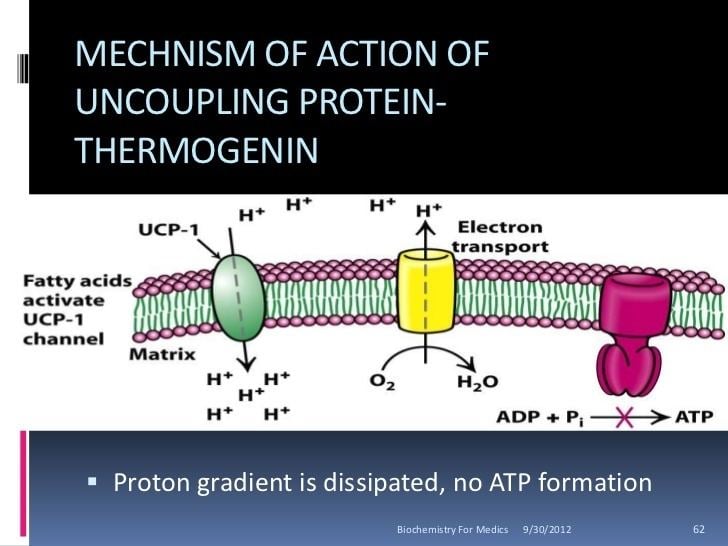
UCP1 is activated in the brown fat cell by fatty acids and inhibited by nucleotides. Fatty acids cause the following signaling cascade: Sympathetic nervous system terminals release Norepinephrine onto a Beta-3 adrenergic receptor on the plasma membrane. This activates adenylyl cyclase, which catalyses the conversion of ATP to cyclic AMP (cAMP). cAMP activates protein kinase A, causing its active C subunits to be freed from its regulatory R subunits. Active protein kinase A, in turn, phosphorylates triacylglycerol lipase, thereby activating it. The lipase converts triacylglycerols into free fatty acids, which activate UCP1, overriding the inhibition caused by purine nucleotides (GDP and ADP). At the termination of thermogenesis, the mitochondria oxidize away the residual fatty acids, UCP1 inactivates and the cell resumes its normal energy-conserving mode.
History
Uncoupling protein 1 was discovered in 1978 and was first cloned in 1988.
Uncoupling protein two (UCP2), a homolog of UCP1, was identified in 1997. UCP2 localizes to a wide variety of tissues, and is thought to be involved in regulating reactive oxygen species (ROS). In the past decade, three additional homologs of UCP1 have been identified, including UCP3, UCP4, and BMCP1 (also known as UCP5).
Clinical Relevance
Methods of delivering UCP1 to cells (e.g. by gene transfer therapy) or methods of its upregulation have been an important line of enquiry in research into the treatment of obesity, due to their ability to dissipate excess metabolic stores.