 | ||
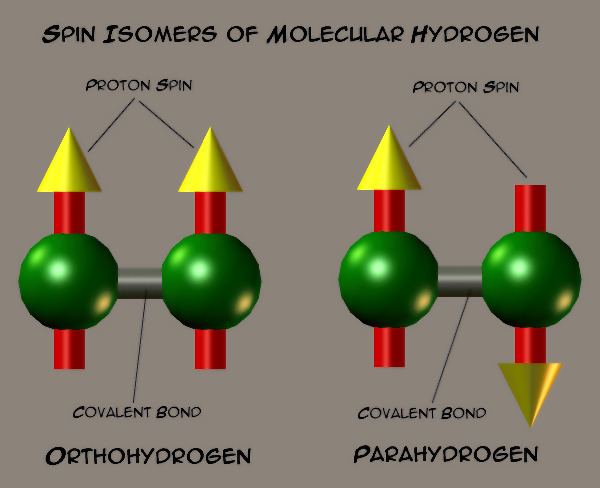
Molecular hydrogen occurs in two isomeric forms, one with its two proton spins aligned parallel (orthohydrogen), the other with its two proton spins aligned antiparallel (parahydrogen). These two forms are often referred to as spin isomers, since they differ not in chemical structure (like most isomers) but rather in nuclear spin state.
Contents
Parahydrogen is in a lower energy state than is orthohydrogen. At room temperature and thermal equilibrium, thermal excitation causes hydrogen to consist of approximately 75% orthohydrogen and 25% parahydrogen. When hydrogen is liquified at low temperature, there is a slow spontaneous transition to a predominantly para ratio, with the released energy having implications for storage. Essentially pure parahydrogen form can be obtained at very low temperatures, but it is not possible to obtain a sample containing more than 75% orthohydrogen by cooling.
Nuclear spin states of H2
Each hydrogen molecule (H2) consists of two hydrogen atoms linked by a covalent bond. If we neglect the small proportion of deuterium and tritium which may be present, each hydrogen atom consists of one proton and one electron. Each proton has an associated magnetic moment, which is associated with the proton's spin of 1/2. In the H2 molecule, the spins of the two hydrogen nuclei (protons) couple to form a triplet state known as orthohydrogen, and a singlet state known as parahydrogen.
The triplet orthohydrogen state has total nuclear spin I = 1 so that the component along a defined axis can have the three values MI = 1, 0, or −1. The corresponding nuclear spin wavefunctions are
The ratio between the ortho and para forms is about 3:1 at standard temperature and pressure – a reflection of the ratio of spin degeneracies. However, if chemical equilibrium between the two forms is established, the para form dominates at low temperatures (approx. 99.8% at 20 K).
Thermal properties
Since protons have spin 1/2, they are fermions and the permutational antisymmetry of the total H2 wavefunction imposes restrictions on the possible rotational states the two forms of H2 can adopt. Orthohydrogen, with symmetric nuclear spin functions, can only have rotational wavefunctions that are antisymmetric with respect to permutation of the two protons, corresponding to odd values of the rotational quantum number J; conversely, parahydrogen with an antisymmetric nuclear spin function, can only have rotational wavefunctions that are symmetric with respect to permutation of the two protons, corresponding to even J. Applying the rigid rotor approximation, the energies and degeneracies of the rotational states are given by:
The rotational partition function is conventionally written as:
However, as long as these two spin isomers are not in equilibrium, it is more useful to write separate partition functions for each:
The factor of 3 in the partition function for orthohydrogen accounts for the spin degeneracy associated with the +1 spin state; when equilibrium between the spin isomers is possible, then a general partition function incorporating this degeneracy difference can be written as:
The molar rotational energies and heat capacities are derived for any of these cases from:
Plots shown here are molar rotational energies and heat capacities for ortho- and parahydrogen, and the "normal" ortho/para (3:1) and equilibrium mixtures:
Because of the antisymmetry-imposed restriction on possible rotational states, orthohydrogen has residual rotational energy at low temperature wherein nearly all the molecules are in the J = 1 state (molecules in the symmetric spin-triplet state cannot fall into the lowest, symmetric rotational state) and possesses nuclear-spin entropy due to the triplet state's threefold degeneracy. The residual energy is significant because the rotational energy levels are relatively widely spaced in H2; the gap between the first two levels when expressed in temperature units is twice the characteristic rotational temperature for H2:
This is the T = 0 intercept seen in the molar energy of orthohydrogen. Since "normal" room-temperature hydrogen is a 3:1 ortho:para mixture, its molar residual rotational energy at low temperature is (3/4) x 2Rθrot = 1091 J/mol, which is somewhat larger than the enthalpy of vaporization of normal hydrogen, 904 J/mol at the boiling point, Tb = 20.369 K. Notably, the boiling points of parahydrogen and normal (3:1) hydrogen are nearly equal; for parahydrogen ∆Hvap = 898 J/mol at Tb = 20.277 K, and it follows that nearly all the residual rotational energy of orthohydrogen is retained in the liquid state.
However, orthohydrogen is thermodynamically unstable at low temperatures and spontaneously converts into parahydrogen. This process lacks any natural de-excitation radiation mode, so it is slow in the absence of a catalyst which can facilitate interconversion of the singlet and triplet spin states. At room temperature, hydrogen contains 75% orthohydrogen, a proportion which the liquefaction process preserves if carried out in the absence of a catalyst like ferric oxide, activated carbon, platinized asbestos, rare earth metals, uranium compounds, chromic oxide, or some nickel compounds to accelerate the conversion of the liquid hydrogen into parahydrogen. Alternatively, additional refrigeration equipment can be used to slowly absorb the heat that the orthohydrogen fraction will (more slowly) release as it spontaneously converts into parahydrogen. If orthohydrogen is not removed from rapidly liquified hydrogen, without a catalyst, the heat released during its decay can boil off as much as 50% of the original liquid.
The two forms of molecular hydrogen were first proposed by Werner Heisenberg and Friedrich Hund in 1927. Taking into account this theoretical framework, pure parahydrogen was first synthesized by Paul Harteck and Karl Friedrich Bonhoeffer in 1929. When Heisenberg was awarded the 1932 Nobel prize in physics for the creation of quantum mechanics, this discovery of the "allotropic forms of hydrogen" was singled out as its most noteworthy application. Modern isolation of pure parahydrogen has since been achieved using rapid in-vacuum deposition of millimeters thick solid parahydrogen (p–H2) samples which are notable for their excellent optical qualities.
Use in NMR
When an excess of parahydrogen is used during hydrogenation reactions (instead of the normal mixture of orthohydrogen to parahydrogen of 3:1), the resultant product exhibits hyperpolarized signals in proton NMR spectra, an effect termed PHIP (Parahydrogen Induced Polarisation) or, equivalently, PASADENA (Parahydrogen And Synthesis Allow Dramatically Enhanced Nuclear Alignment) (named for first recognition of the effect by Bowers and Weitekamp of Caltech), a phenomenon that has been used to study the mechanism of hydrogenation reactions.
Other substances with spin isomers
Other molecules and functional groups containing two hydrogen atoms, such as water and methylene, also have ortho- and para- forms (e.g. orthowater and parawater), but this is of little significance for their thermal properties. Their ortho-para ratios differ from that of dihydrogen.
Molecular oxygen (O
2) also exists in three lower-energy triplet states and one singlet state, as ground-state paramagnetic triplet oxygen and energized highly reactive diamagnetic singlet oxygen. These states arise from the spins of their unpaired electrons, not their protons or nuclei.
