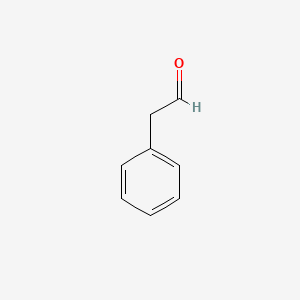
Formula C8H8O | Molar mass 120.15 g/mol Appearance Colorless liquid | |
 | ||
Related 2-phenyl aldehydes | ||
Phenylacetaldehyde is an organic compound used in the synthesis of fragrances and polymers.
Contents
Natural Occurrence

Phenylacetaldehyde occurs extensively in nature because it can be biosynthetically derived from the amino acid phenylalanine. Natural sources of the compound include chocolate, buckwheat, flowers, and communication pheromones from various insect orders.
Fragrances and flavors

The aroma of pure substance can be described as honey-like, sweet, rose, green, grassy and is added to fragrances to impart hyacinth, narcissi, or rose nuances. For similar reasons the compound can sometimes be found in flavored cigarettes and beverages.

Historically, before biotechnology approaches were developed, phenylacetaldehyde was also used to produce phenylalanine via the Strecker reaction as a step in the production of aspartame sweetener.
Polymers
Phenylacetaldehyde is used in the synthesis of polyesters where it serves as a rate-controlling additive during polymerization.
Natural Medicine
Phenylacetaldehyde is responsible for the antibiotic activity of maggot therapy.
Preparation
Phenylacetaldehyde can be obtained via various synthetic routes and precursors. Notable examples include:

Reactivity

Phenylacetaldehyde is often contaminated with polystyrene oxide polymer because of the especial lability of the benzylic alpha proton and the reactivity of the aldehyde. Aldol condensation of the initial dimer gives rise to a range of Michael acceptors and donors.