Appearance colorless oily liquid Molar mass 104.15 g/mol Boiling point 145 °C | Formula C8H8 Density 909 kg/m³ | |
 | ||
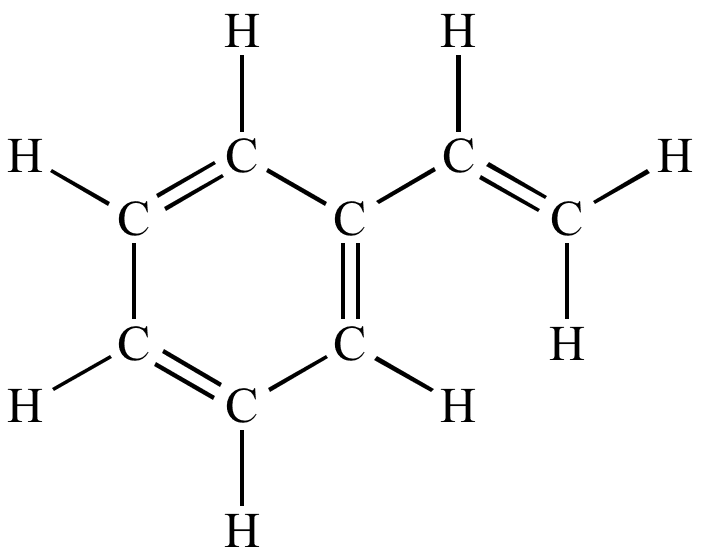
3d styrene derivative of benzene
Styrene, also known as ethenylbenzene, vinylbenzene, and phenylethene, is an organic compound with the chemical formula C6H5CH=CH2. This derivative of benzene is a colorless oily liquid that evaporates easily and has a sweet smell, although high concentrations have a less pleasant odor. Styrene is the precursor to polystyrene and several copolymers. Approximately 25 million tonnes (55 billion pounds) of styrene were produced in 2010.
Contents
- 3d styrene derivative of benzene
- Acrylonitrile butadiene styrene
- Natural occurrence
- History
- Industrial production from ethylbenzene
- From ethylbenzene hydroperoxide
- From toluene and methanol
- From benzene and ethane
- Laboratory synthesis
- Polymerization
- Health effects
- References
Acrylonitrile butadiene styrene
Natural occurrence
Styrene is named for storax balsam, the resin of Liquidambar trees of the Altingiaceae plant family. Styrene occurs naturally in small quantities in some plants and foods (cinnamon, coffee beans, and peanuts), and is also found in coal tar.
History
In 1839, the German apothecary Eduard Simon isolated a volatile oil from the resin (called storax or styrax (Latin)) of the American sweetgum tree (Liquidambar styraciflua). He called the oil "Styrol" (now: "styrene"). He also noticed that when Styrol was exposed to air, light, or heat, it gradually transformed into a hard, rubber-like substance, which he called "Styroloxyd" (styrol oxide, now: "polystyrene"). By 1845, the German chemist August Hofmann and his student John Blyth (1814–1871) had determined Styrol's empirical formula: C8H8. They had also determined that Simon's "Styroloxyd" — which they renamed "Metastyrol" — had the same empirical formula as Styrol. Furthermore, they could obtain Styrol by dry distilling Metastyrol. In 1865, the German chemist Emil Erlenmeyer found that Styrol could form a dimer, and in 1866 the French chemist Marcelin Berthelot stated that Metastyrol was a polymer of Styrol. Meanwhile, other chemists had been investigating another component of storax, namely, cinnamic acid. They had found that cinnamic acid could be decarboxylated to form cinnamène (or cinnamol), which appeared to be Styrol. In 1845, French chemist Emil Kopp suggested that the two compounds were identical, and in 1866, Erlenmeyer suggested that both cinnamol and Styrol might be vinyl benzene. However, the Styrol that was obtained from cinnamic acid seemed different from the Styrol that was obtained by distilling storax resin: the latter was optically active. Eventually, in 1876, the Dutch chemist van 't Hoff resolved the ambiguity: the optical activity of the Styrol that was obtained by distilling storax resin was due to a contaminant.
Industrial production from ethylbenzene

The modern method for production of styrene by dehydrogenation of ethylbenzene was first achieved in the 1930s. The production of styrene increased dramatically during the 1940s, when it was popularized as a feedstock for synthetic rubber. Because it is produced on such a large scale, ethylbenzene in turn prepared on a prodigious scale (by alkylation of benzene with ethylene). Ethylbenzene is mixed in the gas phase with 10–15 times its volume in high-temperature steam, and passed over a solid catalyst bed. Most ethylbenzene dehydrogenation catalysts are based on iron(III) oxide, promoted by several percent potassium oxide or potassium carbonate.
Steam serves several roles in this reaction. It is the source of heat for powering the endothermic reaction, and it removes coke that tends to form on the iron oxide catalyst through the water gas shift reaction. The potassium promoter enhances this decoking reaction. The steam also dilutes the reactant and products, shifting the position of chemical equilibrium towards products. A typical styrene plant consists of two or three reactors in series, which operate under vacuum to enhance the conversion and selectivity. Typical per-pass conversions are ca. 65% for two reactors and 70-75% for three reactors. Selectivity to styrene is 93-97%. The main byproducts are benzene and toluene. Because styrene and ethylbenzene have similar boiling points (145 and 136 °C, respectively), their separation requires tall distillation towers and high return/reflux ratios. At its distillation temperatures, styrene tends to polymerize. To minimize this problem, early styrene plants added elemental sulfur to inhibit the polymerization. During the 1970s, new free radical inhibitors consisting of nitrated phenol-based retarders were developed. More recently, a number of additives have been developed that exhibit superior inhibition against polymerization. However, the nitrated phenols are still widely used because of their relatively low cost. These reagents are added prior to the distillation.
Improving conversion and so reducing the amount of ethylbenzene that must be separated is the chief impetus for researching alternative routes to styrene. Other than the POSM process, none of these routes like obtaining styrene from butadiene have been commercially demonstrated.
From ethylbenzene hydroperoxide
Styrene is also co-produced commercially in a process known as POSM (Lyondell Chemical Company) or SM/PO (Shell) for styrene monomer / propylene oxide. In this process ethylbenzene is treated with oxygen to form the ethylbenzene hydroperoxide. This hydroperoxide is then used to oxidize propylene to propylene oxide. The resulting 1-phenylethanol is dehydrated to give styrene:
From toluene and methanol
Styrene can be produced from toluene and methanol, which are cheaper raw materials than those in the conventional process. This process has suffered from low selectivity associated with the competing decomposition of methanol. Exelus Inc. claims to have developed this process with commercially viable selectivities, at 400-425 °C and atmospheric pressure, by forcing these components through a proprietary zeolitic catalyst. It is reported that an approximately 9:1 mixture of styrene and ethylbenzene is obtained, with a total styrene yield of over 60%.
From benzene and ethane
Another route to styrene involves the reaction of benzene and ethane. This process is being developed by Snamprogetti S.p.A. and Dow. Ethane, along with ethylbenzene, is fed to a dehydrogenation reactor with a catalyst capable of simultaneously producing styrene and ethylene. The dehydrogenation effluent is cooled and separated and the ethylene stream is recycled to the alkylation unit. The process attempts to overcome previous shortcomings in earlier attempts to develop production of styrene from ethane and benzene, such as inefficient recovery of aromatics, production of high levels of heavies and tars, and inefficient separation of hydrogen and ethane. Development of the process is ongoing.
Laboratory synthesis
A laboratory synthesis of styrene entails the decarboxylation of cinnamic acid:
C6H5CH=CHCO2H → :C6H5CH=CH2 + CO2Styrene was first prepared by this method.
Polymerization
The presence of the vinyl group allows styrene to polymerize. Commercially significant products include polystyrene, ABS, styrene-butadiene (SBR) rubber, styrene-butadiene latex, SIS (styrene-isoprene-styrene), S-EB-S (styrene-ethylene/butylene-styrene), styrene-divinylbenzene (S-DVB), styrene-acrylonitrile resin (SAN), and unsaturated polyesters used in resins and thermosetting compounds. These materials are used in rubber, plastic, insulation, fiberglass, pipes, automobile and boat parts, food containers, and carpet backing.
Health effects
Styrene is regarded as a "hazardous chemical", especially in case of eye contact, but also in case of skin contact, of ingestion and of inhalation, according to several sources. Styrene is largely metabolized into styrene oxide in humans, resulting from oxidation by cytochrome P450. Styrene oxide is considered toxic, mutagenic, and possibly carcinogenic. Styrene oxide is subsequently hydrolyzed in vivo to styrene glycol by the enzyme epoxide hydrolase. The U.S. Environmental Protection Agency (EPA) has described styrene to be "a suspected toxin to the gastrointestinal tract, kidney, and respiratory system, among others". On 10 June 2011, the U.S. National Toxicology Program has described styrene as "reasonably anticipated to be a human carcinogen". However, a STATS author describes a review that was done on scientific literature and concluded that "The available epidemiologic evidence does not support a causal relationship between styrene exposure and any type of human cancer". Despite this claim, work has been done by Danish researchers to investigate the relationship between occupational exposure to styrene and cancer. They concluded, "The findings have to be interpreted with caution, due to the company based exposure assessment, but the possible association between exposures in the reinforced plastics industry, mainly styrene, and degenerative disorders of the nervous system and pancreatic cancer, deserves attention". The Danish EPA recently concluded that the styrene data do not support a cancer concern for styrene.
Various regulatory bodies refer to styrene, in various contexts, as a possible or potential human carcinogen. The International Agency for Research on Cancer considers styrene to be "possibly carcinogenic to humans". Chronic exposure to styrene leads to tiredness/lethargy, memory deficits, headaches and vertigo.
The U.S. EPA does not have a cancer classification for styrene, but is has been the subject of their Integrated Risk Information System (IRIS) program. The U.S. National Toxicology Program of the U.S. Department of Health and Human Services has determined that styrene is "reasonably anticipated to be a human carcinogen".
