Formula H2SO5 Density 2.29 g/cm³ | Molar mass 114.04 g/mol Appearance White crystals | |
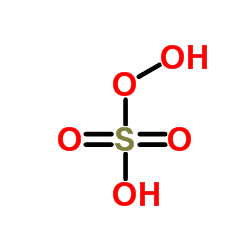 | ||
Peroxymonosulfuric acid, (H2SO5), also known as persulfuric acid, peroxysulfuric acid, or Caro's acid, is a liquid at room temperature. In this acid, the S(VI) center adopts its characteristic tetrahedral geometry; the connectivity is indicated by the formula HO–O–S(O)2–OH. It is one of the strongest oxidants known (E0 = +2.51 V) and is highly explosive.
Contents

H2SO5 is sometimes confused with H2S2O8, known as peroxydisulfuric acid. The disulfuric acid, which appears to be more widely used as its alkali metal salts, has the structure HO–S(O)2–O–O–S(O)2–OH.
History
H2SO5 was first described in 1898 by Heinrich Caro, after whom it is named.
Synthesis and production
The laboratory scale preparation of Caro's acid involve the combination of chlorosulfuric acid and hydrogen peroxide.
Large scale production of Caro's acid is usually done on site, due to its instability. According to the patent by Martin, Caro's acid is produced by reacting >85% sulfuric acid and <50% hydrogen peroxide ("Piranha solution").
Uses in industry
H2SO5 has been used for a variety of disinfectant and cleaning applications, e.g., swimming pool treatment and denture cleaning. Alkali metal salts of H2SO5 show promise for the delignification of wood.
Ammonium, sodium, and potassium salts of H2SO5 are used in the plastic industry as polymerization initiators, etchants, desizing agents, soil conditioner, and for decolorizing and deodorizing oils.
Potassium peroxymonosulfate, KHSO5, is the potassium acid salt of peroxymonosulfuric acid. It is widely used as an oxidizing agent.
Dangers
Pure Caro's acid is highly explosive. Explosions have been reported at Brown University and Sun Oil. As with all strong oxidizing agents, peroxysulfuric acid should be kept away from organic compounds such as ethers and ketones because of its ability to peroxidize the compound, creating a highly unstable molecule such as acetone peroxide.
