Group Group II (ssDNA) Scientific name Parvovirus Rank Genus | Family Parvoviridae Higher classification Parvoviridae | |
 | ||
Similar Canine parvovirus, Parvoviridae, Feline distemper, Porcine parvovirus, Bordetella | ||
Dog parvovirus parvovirus in dogs
Parvovirus is the common name applied to all the viruses in the Parvoviridae taxonomic family. The Parvoviridae family has two subfamilies; the Parvovirinae (vertebrate viruses) and the Densovirinae (invertebrate viruses). Different examples can be given for the subfamily Parvovirinae but the most common is Dependovirus, which only work with a helper virus such as adenovirus. Other viruses that can infect without helper viruses are called as autonomous parvoviruses.
Contents
- Dog parvovirus parvovirus in dogs
- How to help dogs with parvovirus
- History
- Structure
- Dependoviruses
- Autonomous Parvoviruses
- Disease information on Parvoviridae
- Diseases caused by members of the Parvoviridae family
- Replication as disease vector
- Use of HeLa cells in parvovirus testing
- Canine and feline
- Management and therapy
- References
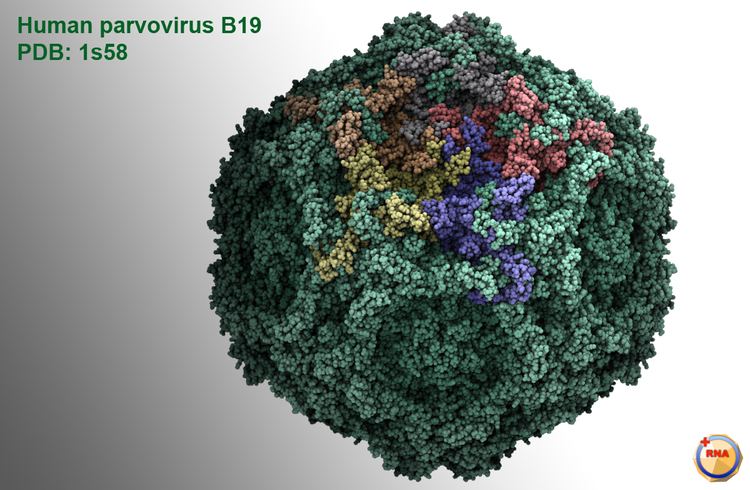
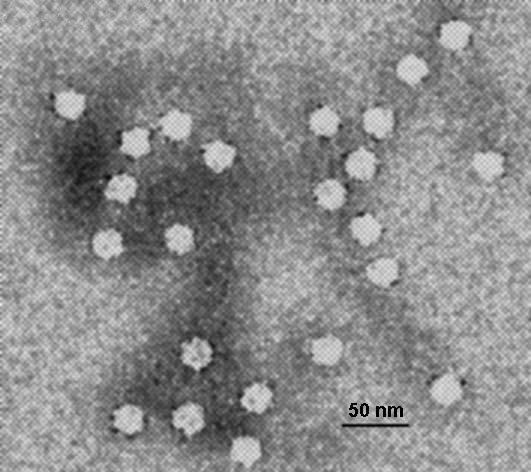
Parvoviruses are linear, non-segmented single-stranded DNA viruses, with an average genome size of 5000 nucleotides. They are classified as group II viruses in the Baltimore classification of viruses. Parvoviruses are among the smallest viruses (hence the name, from Latin parvus meaning small) and are 18–28 nm in diameter.
Parvoviruses can cause disease in some animals, including starfish and humans. Because the viruses require actively dividing cells to replicate, the type of tissue infected varies with the age of the animal. The gastrointestinal tract and lymphatic system can be affected at any age, leading to vomiting, diarrhea and immunosuppression but cerebellar hypoplasia is only seen in cats that were infected in the womb or at less than two weeks of age, and disease of the myocardium is seen in puppies infected between the ages of three and eight weeks.
How to help dogs with parvovirus
History
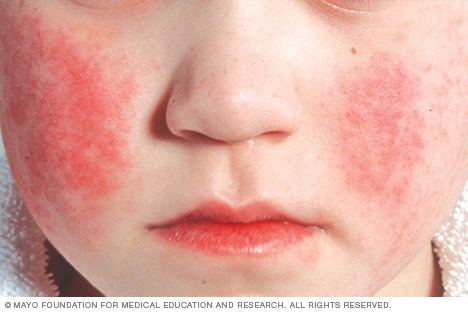
Perhaps due to their extremely small size, parvoviruses were only recently discovered. Dependoviruses, the first parvoviruses to be discovered, were first isolated in the 1960s. Parvovirus B19, the first known parvovirus to infect humans, was discovered in London by Australian virologist Yvonne Cossart in 1974. Cossart and her group were focused on hepatitis B and were processing blood samples when they discovered a number of "false positives" later identified as parvovirus B19. The virus is named for the patient code of one of the blood bank samples involved in the discovery.
Structure
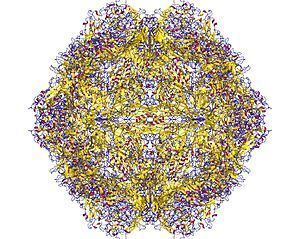
The viral capsid of a parvovirus is made up of two to four proteins, known as VP1-4 that form an icosahedral symmetry that is resistant to acids, bases, solvents and temperature up to 50 °C (122 degrees Fahrenheit). The capsid is also constructed from 60 protein molecules and one of them creates the majority of the viral capsid structure. Parvoviruses do not have envelopes and are thus considered "naked" viruses. In addition, the shape of the virion is roughly spherical, with surface protrusions and canyons.
Inside the capsid is a linear single-stranded DNA genome in the size range 4–6 kb so the small genome of parvovirus can encode only a few proteins. At the 5’ and 3’ ends of this genome are short complementary sequences of approximately 120 to 250 nucleotides, that form secondary structures as hairpins for example inverted terminal repeats (ITRs which are two identical secondary structures at the termini) or unique sequences at the termini (there are two unique and different secondary structures at each end of the DNA) and are essential for viral genome replication mechanism called rolling-hairpin replication.
Dependoviruses
Dependoviruses require helper viruses (e.g. herpesviruses) to replicate. They are also perfect candidates as gene vectors. They are utilized to investigate genes in cell cultures of the proteins which are encoded by those genes via mass production method or manipulated as probable vectors to examine genes in the cells of patients for diagnosis and treatment of several genetic diseases and cancers. The biggest advantage for such applications is that they are not known to cause any diseases.
Autonomous Parvoviruses
Autonomous Parvoviruses do not require a helper virus like dependoviruses. The virus B19 was discovered in blood serum and infects red blood cell precursors. Some infections do not result in visible infection, while some manifest with visible effects, such as fifth disease (erythema infectiosum), which can give children a ‘slapped-cheek’ appearance.
Disease information on Parvoviridae
The remainder of this article discusses the disease-causing Parvoviridae.
Diseases caused by members of the Parvoviridae family
Parvovirus B19, which causes fifth disease in humans, is a member of the Erythrovirus genus of the Parvoviridae.
Prior to 2014, it was also the name applied to a genus within the subfamily Parvovirinae, but this has been amended to genus Protoparvovirus to avoid confusion between taxonomic levels. Parvoviruses that infect vertebrate hosts make up the subfamily Parvovirinae, while those that infect arthropods (currently only known to infect insects or shrimp) make up the subfamily Densovirinae.
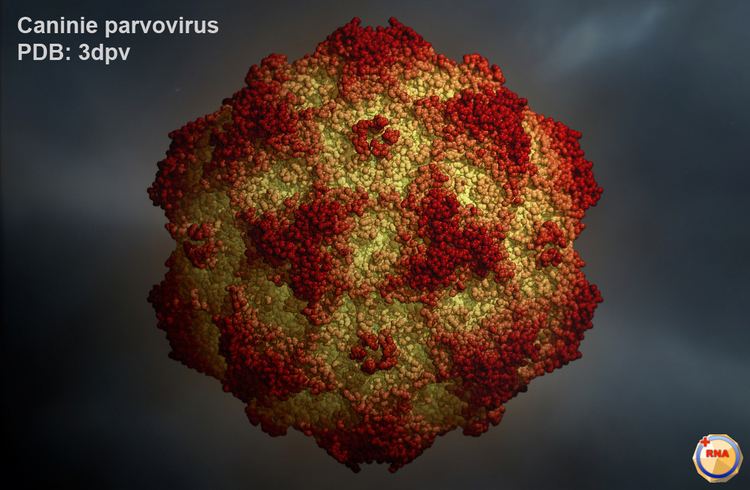
Many mammalian species sustain infection by multiple parvoviruses. Parvoviruses tend to be specific about the species of animal they will infect, but this is a somewhat flexible characteristic. Thus, all isolates of canine parvovirus affect dogs, wolves, and foxes, but only some of them will infect cats.
Humans can be infected by viruses from five of the eight genera in the subfamily Parvovirinae: i) Bocaparvovirus (e.g. human bocavirus 1), ii) Dependoparvovirus (e.g. adeno-associated virus 2), iii) Erythroparvovirus (e.g. parvovirus B19), iv) Protoparvovirus (e.g. bufavirus 1a) and v) Tetraparvovirus (e.g. human parv4 G1). As of 2014, there were no known human viruses in the remaining three recognized genera: vi) Amdoparvovirus (e.g. Aleutian mink disease virus), vii) Aveparvovirus (e.g. chicken parvovirus) and viii) Copiparvovirus (e.g. bovine parvovirus 2).
Canine parvovirus is a particularly deadly disease among young puppies, about 80% fatal, causing gastrointestinal tract damage and dehydration as well as a cardiac syndrome in very young animals. It is spread by contact with an infected dog's feces. Symptoms include lethargy, severe diarrhea, fever, vomiting, loss of appetite, and dehydration.
Mouse parvovirus 1, however, causes no symptoms but can contaminate immunology experiments in biological research laboratories.
Porcine parvovirus causes a reproductive disease in swine known as SMEDI, which stands for stillbirth, mummification, embryonic death, and infertility.
Feline panleukopenia is common in kittens and causes fever, low white blood cell count, diarrhea, and death. Infection of the cat fetus and kittens less than two weeks old causes cerebellar hypoplasia.
Mink enteritis virus is similar in effect to feline panleukopenia, except that it does not cause cerebellar hypoplasia. A different parvovirus causes Aleutian Disease in mink and other mustelids, characterized by lymphadenopathy, splenomegaly, glomerulonephritis, anemia, and death.
Dogs, cats and swine can be vaccinated against parvovirus.
Replication as disease vector
To enter host cells, parvoviruses bind to a sialic acid-bearing cell surface receptor. Penetration into the cytoplasm is mediated by a phospholipase A2 activity carried on the amino-terminal peptide of the capsid VP1 polypeptide. Once in the cytoplasm, the intact virus is translocated to the nucleus prior to uncoating. Transcription only initiates when the host cell enters S-phase under its own cell cycle control, at which time the cell's replication machinery converts the incoming single strand into a duplex transcription template, allowing synthesis of mRNAs encoding the non-structural proteins, NS1 and NS2. The mRNAs are transported out of the nucleus into the cytoplasm where the host ribosomes translate them into viral proteins. Viral DNA replication proceeds through a series of monomeric and concatemeric duplex intermediates by a unidirectional strand-displacement mechanism that is mediated by components of the host replication fork, aided and orchestrated by the viral NS1 polypeptide. NS1 also transactivates an internal transcriptional promoter that directs synthesis of the structural VP polypeptides. Once assembled capsids are available, replication shifts from synthesizing duplex DNA to displacement of progeny single strands, which are typically negative-sense and are packaged in a 3'-to-5' direction into preformed particles within the nucleus. Mature virions may be released from infected cells prior to cell lysis, which promotes rapid transmission of the virus, but if this fails then the virus is released at cell lysis.
Unlike most other DNA viruses, parvoviruses are unable to activate DNA synthesis in host cells. Thus, in order for viral replication to take place the infected cells must be non-quiescent (i.e. must be actively mitotic). Their inability to force host cells into S-phase means that parvoviruses are non-tumorigenic. Indeed, they are commonly oncolytic, showing a strong tendency to replicate preferentially in cells with transformed phenotypes.
Use of HeLa cells in parvovirus testing
Testing for how feline parvovirus and canine parvovirus infect cells and what pathways are taken; scientists used cat cells, mouse cells, cat and mouse hybrid cells, mink cells, dog cells, human cells, and HeLa cells. Both feline parvovirus and canine parvovirus enter their hosts, follow specific pathways, and infect at certain parts of cells before infecting major organs. Parvoviruses are specific viruses that are characterized by which receptors they attack. Testing found that parvovirus infects carnivorous animals through the oropharyngeal pathway. Parvovirus infects the oropharyngeal cells that come in immediate contact with the virus. It contains a plasmid that infects and binds to transferrin receptors, a glycoprotein, on the plasma membrane. The parvovirus plasmid is stored in a small non-enveloped capsid. Once oropharyngeal cells become infected the virus spreads to dividing lymph cells and continues to work to the bone marrow and spread to target organs through blood.
Testing of HeLa cells and human cells to exposure of both feline parvovirus and canine parvovirus resulted in infections of the cells at human transferrin receptors. When antibodies and parvovirus samples were added at the same time to human cells and HeLa cells it was found that no infection would take place; experiments showed that both human cells and HeLa cells have transferrin receptors but there is no evidence of humans contracting parvovirus.
Certain chromosomes in cells show more susceptibility to parvovirus than others. Testing of feline parvovirus on cat cells and cat mouse hybrid cells found cultures with cells having the highest concentrations of the C2 chromosome were the most highly infected cells. Slight mutations of binding sites were found to slow down or completely stop the infection of the given parvovirus; whereas cells that are naturally missing the receptors or are mutants lacking them cannot be mutated. Both feline parvovirus and canine parvovirus express plasticity during cellular infection. Although transferrin receptors may be limited on cell surfaces the parvovirus will find available transferrin receptors and will use different pathways to gain entry to the cell. Unlike plasma membrane infection plasticity, all strains of parvovirus show related routes to the cell nucleus.
Canine and feline
Canine parvovirus is a mutant strain of feline parvovirus. A very specific mutation is necessary for the virus to change species of infection. The mutation affects capsid proteins of feline parvovirus giving it the ability to infect dogs. Both forms of the virus are very similar, so once the mutation has occurred, canine parvovirus is still able to infect cats. The canine parvovirus has the tradeoff of gaining the ability to infect canine cells while becoming less effective at infecting feline cells. Both feline parvovirus and canine parvovirus bind to and infect the transferrin receptors, but both have different sequences in the cells and animals. Infection by both feline parvovirus and canine parvovirus are relatively quick; but because of constant mutation of canine parvovirus, canine parvovirus has a slower infection time than feline parvovirus. Studies of other strains of mutated canine parvovirus have revealed that changes in the viral capsid by just one protein can be fatal to the virus. Deleterious mutations have been noted to lead to inability to bind to transferrin receptors, bind to non- receptive parts of the cell membrane, and identification of the virus by the host’s antibody cells.
Management and therapy
Currently there is no vaccine to prevent infection by parvoviruses, but recently the virus's capsid proteins, which are noninfectious molecules, have been suggested acting as antigens for improving of vaccines.
Antivirals and human immunoglobulin sourced treatments are usually for relief of symptoms. Utilizing immunoglobulins is a logical solution for treatment as neutralizing antibodies because a majority of adults have been in danger from the parvoviruses especially B19 virus.
