 | ||
The nitrogen-vacancy center (N-V center) is one of numerous point defects in diamond. Its most explored and useful property is photoluminescence, which can be easily detected from an individual N-V center, especially those in the negative charge state (N-V−). Electron spins at N-V centers, localized at atomic scales, can be manipulated at room temperature by applying a magnetic field, electric field, microwave radiation or light, or a combination, resulting in sharp resonances in the intensity and wavelength of the photoluminescence. These resonances can be explained in terms of electron spin related phenomena such as quantum entanglement, spin-orbit interaction and Rabi oscillations, and analysed using advanced quantum optics theory. An individual N-V center can be viewed as a basic unit of a quantum computer, and it has potential applications in novel, more efficient fields of electronics and computational science including quantum cryptography and spintronics.
Contents
Structure
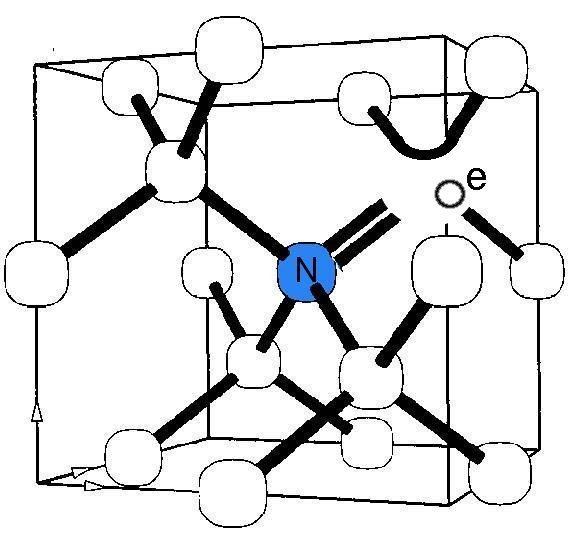
The nitrogen-vacancy center is a point defect in the diamond lattice. It consists of a nearest-neighbor pair of a nitrogen atom, which substitutes for a carbon atom, and a lattice vacancy.
Two charge states of this defect, neutral N-V0 and negative N-V−, are known from spectroscopic studies using optical absorption, photoluminescence (PL), electron paramagnetic resonance (EPR) and optically detected magnetic resonance (ODMR), which can be viewed as a hybrid of PL and EPR; most details of the structure originate from EPR. A nitrogen atom has five valence electrons. Three of them covalently bond to the carbon atoms and two remain non-bonded and are called a lone pair. The vacancy has three unpaired electrons. Two of them make a quasi covalent bond and one remains unpaired. The overall symmetry, however, is axial (trigonal C3V); one can visualize this by imagining the three unpaired vacancy electrons continuously exchanging their roles.
The N-V0 thus has one unpaired electron and is paramagnetic. However, despite extensive efforts, electron paramagnetic resonance signals from N-V0 avoided detection for decades until 2008. Optical excitation is required to bring the N-V0 defect into the EPR-detectable excited state; the signals from the ground state are presumably too broad for EPR detection.
The N-V0 centers can be converted into N-V- by changing the Fermi level position. This can be achieved by applying external voltage to a p-n junction made from doped diamond, e.g., in a Schottky diode.
In the negative charge state N-V−, an extra electron is located at the vacancy site forming a spin S=1 pair with one of the vacancy electrons. As in N-V0, the vacancy electrons are "exchanging roles" preserving the overall trigonal symmetry. This N-V− state is what is commonly, and somewhat incorrectly, called "the nitrogen-vacancy center". The neutral state has not yet been explored for spin manipulations.
The N-V centers are randomly oriented within a diamond crystal. Ion implantation techniques can enable their artificial creation in predetermined positions.
Production
Nitrogen-vacancy centers are typically produced from single substitutional nitrogen centers (called C or P1 centers in diamond literature) by irradiation followed by annealing at temperatures above 700 °C. A wide range of high-energy particles are suitable for such irradiation, including electrons, protons, neutrons, ions, and gamma photons. Irradiation produces lattice vacancies, which are a part of N-V centers. Those vacancies are immobile at room temperature, and annealing is required to move them. Single substitutional nitrogen produces strain in the diamond lattice; it therefore efficiently captures moving vacancies, producing the N-V centers.
During chemical vapor deposition of diamond, a small fraction of single substitutional nitrogen impurity (typically <0.5%) traps vacancies generated as a result of the plasma synthesis. Such nitrogen-vacancy centers are preferentially aligned to the growth direction.
Diamond is notorious for having a relatively large lattice strain. Strain splits and shifts optical transitions from individual centers resulting in broad lines in the ensembles of centers. Special care is taken to produce extremely sharp N-V lines (line width ~10 MHz) required for most experiments: high-quality, pure natural or better synthetic diamonds (type IIa) are selected. Many of them already have sufficient concentrations of grown-in N-V centers and are suitable for applications. If not, they are irradiated by high-energy particles and annealed. Selection of a certain irradiation dose allows tuning the concentration of produced N-V centers such that individual N-V centers are separated by micrometre-large distances. Then, individual N-V centers can be studied with standard optical microscopes or, better, near-field scanning optical microscopes having sub-micrometre resolution.
Basic optical properties
N-V− centers emit bright red light which can be conveniently excited by visible light sources, such as argon or krypton lasers, frequency doubled Nd:YAG lasers, dye lasers, or He-Ne lasers. Excitation can also be achieved at energies below that of zero phonon emission. Laser illumination, however, also converts some N-V− into N-V0 centers. Emission is very quick (relaxation time ~10 ns). At room temperature, no sharp peaks are observed because of the thermal broadening. However, cooling the N-V− centers with liquid nitrogen or liquid helium dramatically narrows the lines down to a width of a few megahertz.
An important property of the luminescence from individual N-V− centers is its high temporal stability. Whereas many single-molecular emitters bleach after emission of 106–108 photons, no bleaching is observed for the N-V centers at room temperature.
Because of these properties, the ideal technique to address the N-V centers is confocal microscopy, both at room temperature and at low temperature. In particular, low temperature operation is required to specifically address only the zero-phonon line (ZPL).
Spin dynamics
Thinking of the N-V− center as a multielectronic system, we can draw the diagram in the figure at right, where the states are labeled according to their symmetry and with a left superscript that indicates with a 3 if it is a triplet (S=1) and with a 0 if it is a singlet (S=0). It is well accepted today that we have two triplet states and two intermediate singlet states.
The optical excitations conserve the spin state, but there is a high probability of the states
Some authors explain the dynamics of the N-V− center by admitting that the transition from
Potential applications
The spectral shape and intensity of the optical signals from the N-V− centers are sensitive to external perturbation, such as temperature, strain, electric and magnetic field. However, the use of spectral shape for sensing those perturbation is impractical, as the diamond would have to be cooled to cryogenic temperatures to sharpen the N-V− signals. A more realistic approach is to use luminescence intensity (rather than lineshape), which exhibits a sharp resonance when a microwave frequency is applied to diamond that matches the splitting of the ground state levels. The resulting optically detected magnetic resonance signals are sharp even at room temperature, and can be used in miniature sensors. Such sensors can detect magnetic fields of a few nanotesla or electric fields of about 10 V/cm at kilohertz frequencies after 100 seconds of averaging. This sensitivity allows detecting a magnetic or electric field produced by a single electron located tens of nanometers away from an N-V− center.
Using the same mechanism, the N-V− centers were employed in scanning thermal microscopy to measure high-resolution spatial maps of temperature and thermal conductivity (see image).
Another possible use the N-V− centers is as a detector to measure the full mechanical stress tensor in the bulk of the crystal. For this application, the stress-induced splitting of the zero-phonon-line is exploited, and its polarization properties. A robust frequency-modulated radio receiver using the electron-spin-dependent photoluminescence that operated up to 350 °C demonstrates the possibility for use in extreme conditions.
In addition to the quantum optical applications, luminescence from the N-V− centers can be applied for imaging biological processes, such as fluid flow in living cells. This application relies on good compatibility of diamond nano-particles with the living cells and on favorable properties of photoluminescence from the N-V− centers (strong intensity, easy excitation and detection, temporal stability, etc.). Compared with large single-crystal diamonds, nanodiamonds are cheap (about 1 USD per gram) and available from various suppliers. N-V− centers are produced in diamond powders with sub-micrometre particle size using the standard process of irradiation and annealing described above. Those nanodiamonds are introduced in a cell, and their luminescence is monitored using a standard fluorescence microscope.
Further N-V− center has been hypothesized to be a potential bio-mimetic system for emulating radical pair spin dynamics of the avian compass.
Stimulated emission from the N-V− center has been demonstrated, though it could be achieved only from the phonon side-band (i.e. broadband light) and not from the ZPL. For this purpose the center has to be excited at wavelength longer than ~650 nm, as higher-energy excitation ionizes the center.
Historical remarks
The microscopic model and most optical properties of ensembles of the N-V− centers have been firmly established in the 1970s based on the optical measurements combined with uniaxial stress and on the electron paramagnetic resonance. However, a minor error in EPR results (it was assumed that illumination is required to observe N-V− EPR signals) resulted in the incorrect multiplicity assignments in the energy level structure. In 1991 it was shown that EPR can be observed without illumination, which established the energy level scheme shown above. The magnetic splitting in the excited state has been measured only recently.
The characterization of single N-V− centers has become a very competitive field nowadays, with many dozens of papers published in the most prestigious scientific journals. One of the first results was reported back in 1997. In that paper, it was demonstrated that the fluorescence of single N-V− centers can be detected by room-temperature fluorescence microscopy and that the defect shows perfect photostability. Also one of the outstanding properties of the N-V center was demonstrated, namely room-temperature optically detected magnetic resonance.
