CAS Number 50700-49-7 IUPHAR/BPS 6299 UNII G6S9BN13TI CAS ID 50700-49-7 | PubChem CID 39763 ChemSpider 36356 Molar mass 149.147 g/mol | |
 | ||
Synonyms N-Acetyl-p-benzoquinone imine; N-Acetylimidoquinone | ||
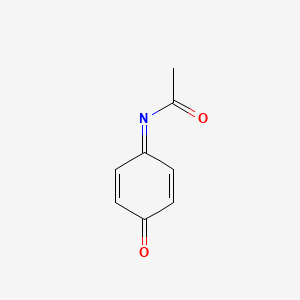
NAPQI (N-acetyl-p-benzoquinone imine) is a toxic byproduct produced during the xenobiotic metabolism of the analgesic paracetamol (acetaminophen). It is normally produced only in small amounts, and then almost immediately detoxified in the liver.
Contents

However, under some conditions in which NAPQI is not effectively detoxified (usually in case of paracetamol overdose), it causes severe damage to the liver. This becomes apparent 3–4 days after ingestion and may result in death from fulminant liver failure several days after the overdose.

Metabolism

In adults, the primary metabolic pathway for paracetamol is glucuronidation. This yields a relatively non-toxic metabolite, which is excreted into bile and passed out of the body. A small amount of the drug is metabolized via the cytochrome P-450 pathway (to be specific, CYP3A4 and CYP2E1) into NAPQI, which is extremely toxic to liver tissue, as well as being a strong biochemical oxidizer. In an average adult, only a small amount (approximately 10% of a therapeutic paracetamol dose) of NAPQI is produced, which is inactivated by conjugation with glutathione. The amount of NAPQI produced differs in certain populations.

The minimum dosage at which paracetamol causes toxicity usually is 7.5 to 10g in the average person. The lethal dose is usually between 10 g and 15 g. Concurrent alcohol intake lowers these thresholds significantly. Chronic alcoholics may be more susceptible to adverse effects due to reduced glutathione levels. Other populations may experience effects at lower or higher dosages depending on differences in P-450 enzyme activity and other factors which affect the amount of NAPQI produced. In general, however, the primary concern is accidental or intentional paracetamol overdose. Since the drug is available over the counter in most jurisdictions, it is a common choice for suicide attempts.

When a toxic dose of paracetamol is ingested, the normal glucuronide pathway is saturated and large amounts of NAPQI are produced. Liver reserves of glutathione are depleted by conjugation with this excess NAPQI. The mechanism by which toxicity results is complex, but is believed to involve reaction between unconjugated NAPQI and critical proteins as well as increased susceptibility to oxidative stress caused by the depletion of glutathione.
Poisoning

The prognosis is good for paracetamol overdoses if treatment is initiated up to 8 hours after the drug has been taken. Most hospitals stock the antidote (acetylcysteine), which replenishes the liver's supply of glutathione, allowing the NAPQI to be metabolized safely. Without early administration of the antidote, fulminant liver failure follows, often in combination with kidney failure, and death generally occurs within several days.
Antidote
The preferred antidote for NAPQI poisoning (usually secondary to paracetamol poisoning) is N-acetylcysteine. It can be given in large amounts without side-effects. The amino acid methionine can also be used effectively as an antidote for NAPQI toxicity. The antidote should be held off to get blood paracetamol levels if the levels can be obtained with in 8 hours post-ingestion. There is little indication of giving the anti-dote before 8 hours.
