Boiling point 101 °C Molar mass 98.186 g/mol | Formula C7H14 Density 770 kg/m³ Melting point -126.3 °C | |
 | ||
Double newman diagram for methylcyclohexane organic chemistry khan academy
Methylcyclohexane is an organic compound with the molecular formula is CH3C6H11. Classified as saturated hydrocarbon, it is a colourless liquid with a faint odor. Methylcyclohexane is used as a solvent. It is mainly converted in naphtha reformers to toluene.
Contents
- Double newman diagram for methylcyclohexane organic chemistry khan academy
- Production and use
- Solvent
- Structure
- Flammability and toxicity
- References

Production and use
It can be also produced by hydrogenation of toluene:
CH3C6H5 + 3 H2 → CH3C6H11
Methylcyclohexane, as a component of a mixture, is usually dehydrogenated to toluene, which increases the octane rating of gasoline.
It is also one of a host substances in jet fuel surrogate blends, e.g., for Jet A fuel.
Solvent

Methylcyclohexane has no particular applications, although it used as an organic solvent, with properties similar to related saturated hydrocarbons such as heptane.
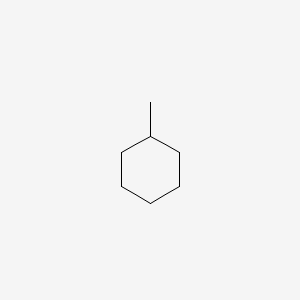
Structure

Methylcyclohexane is a monosubstituted cyclohexane because it has one branching via the attachment of one methyl group on one carbon of the cyclohexane ring. Like all cyclohexanes, it can interconvert rapidly between two chair conformers. The lowest energy form of this monosubstituted methylcyclohexane occurs when the methyl group occupies an equatorial rather than an axial position. This equilibrium is embodied in the concept of A value. In the axial position, the methyl group experiences steric crowding (steric strain) because of the presence of axial hydrogen atoms on the same side of the ring (known as the 1,3-diaxial interactions). There are two such interactions, with each pairwise methyl/hydrogen combination contributing approximately 7.61 kJ/mol of strain energy. The equatorial conformation experiences no such interaction, and so it is the energetically favored conformation.
Flammability and toxicity
Like all hydrocarbons, methylcyclohexane is flammable.
Furthermore, it is considered "very toxic to aquatic life". Note, while methylcyclohexane is a substructure of 4-methylcyclohexanemethanol (MCHM), it is distinct in its physical, chemical, and biological (ecologic, metabolic, and toxicologic) properties.


