EC number 3.2.1.17 ExPASy NiceZyme view | CAS number 9001-63-2 | |
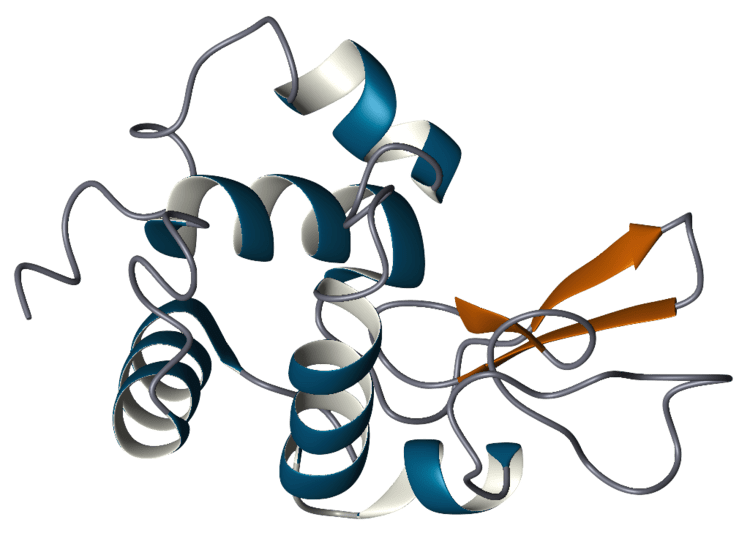 | ||
Lysozyme
Lysozyme, also known as muramidase or N-acetylmuramide glycanhydrolase is an antimicrobial enyzme produced by animals that forms part of the innate immune system. Lysozyme is a glycoside hydrolase which catalyze the hydrolysis of 1,4-beta-linkages between N-acetylmuramic acid and N-acetyl-D-glucosamine residues in peptidoglycans which is the major component of gram-positive bacterial cell wall. This hydrolysis in turn compromises the integrity of bacterial cell walls causing lysis of the bacteria.
Contents
- Lysozyme
- Lysozyme enzyme
- Function and mechanism
- Phillips
- Koshland
- Inhibition
- Enzyme conformation changes
- Role in disease and therapy
- Chemical synthesis
- Other applications
- History
- References
Lysozyme is abundant in a number of secretions, such as tears, saliva, human milk, and mucus. It is also present in cytoplasmic granules of the macrophages and the polymorphonuclear neutrophils (PMNs). Large amounts of lysozyme can be found in egg white. C-type lysozymes are closely related to alpha-lactalbumin in sequence and structure, making them part of the same family. In humans, the lysozyme enzyme is encoded by the LYZ gene.
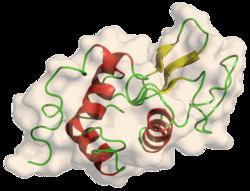
Lysozyme is thermal stable, whose melting point can reach up to 72 ℃ at pH 5.0. But in human milk it loses activity very fast at that temparature. Its isoelectric point is 11.35. In a large range of pH (6-9) lysozyme can survive.
Lysozyme enzyme
Function and mechanism
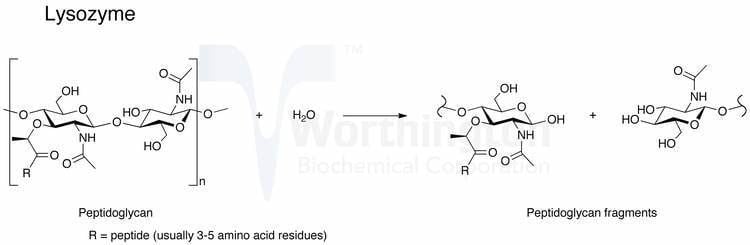
The enzyme functions by attacking, hydrolyzing, and breaking glycosidic bonds in peptidoglycans. The enzyme can also break glycosidic bonds in chitin, although not as effective as true chitinases. The ability to break down both oligosaccharides suggests a similar mechanism between the breakdown of the two molecules.

Lysozymes active site binds the peptidoglycan molecule in the prominent cleft between its two domains. It attacks peptidoglyans (found in the cell walls of bacteria, especially Gram-positive bacteria), its natural substrate, between N-acetylmuramic acid(NAM) and the fourth carbon atom of N-acetylglucosamine(NAG).
Shorter saccharides like tetrasaccharide have also shown to be viable substrates but via an intermediate with a longer chain. Chitin has also been show to be a viable lysozyme substrate. Artificial substrates have also been developed and used in lysozyme.
Phillips
The Phillips Mechanism proposed that the enzyme's catalytic power came from both steric strain on the bound substrate and electrostatic stabilization of the oxo-carbenium intermediate. From x-ray crystallography data, Phillips proposed the lysozyme's active site binds to a hexasaccharide. The lysozyme distorts the fourth sugar in hexasaccharide into a half-chair conformation. In this stressed state, the glycosidic bond is more easily broken. An ionic intermediate containing an oxo-carbenium is created as a result of the the glycosidic bond breaking. Thus distortion causing the substrate molecule to adopt a strained conformation similar to that of the transition state will lower the energy barrier of the reaction.
This oxo-carbonium intermediate was also proposed to be electrostatically stabilized by Arieh Warshel in 1978. Residues in the active site, such as aspartate and glutamate are able to stabilize this intermediate. The electrostatic stabilization argument was based on comparison to bulk water, the reorientation of water dipoles can cancel out the stabilizing energy of charge interaction. In Warshel's model, the enzyme acts as a super-sovlent, which fixes the orientation of ion pairs and provides super-solvation (very good stabilization of ion pairs), and especially lower the energy when to ions are close to each other.
The rate-determining step(RDS) in this mechanism is related to formation of the oxo-carbenium intermediate. There were some contradictory results to indicate the exact RDS. By tracing the formation of product (p-nitrophenol), it was discovered that the RDS can change over different temperatures, which was a reason for those contradictory results. At a higher temperature the RDS is formation of glycosyl enzyme intermediate and at a lower temperature the break down of that intermediate.
Koshland
In an early debate in 1969, Dahlquist proposed a covalent mechanism for lysozyme based on kinetic isotope effect, but for a long time the ionic mechanism was more accepted. In 2001, a revised mechanism was proposed by Vocadlo via a covalent but not ionic intermediate. Evidence from ESI-MS analysis indicated a covalent intermediate. A 2-Fluorine substituted substrate was used to lower reaction rate and accumulate an intermediate for characterization. The amino acid side-chains glutamic acid 35 (Glu35) and aspartate 52 (Asp52) have been found to be critical to the activity of this enzyme. Glu35 acts as a proton donor to the glycosidic bond, cleaving the C-O bond in the substrate, whereas Asp52 acts as a nucleophile to generate a glycosyl enzyme intermediate. The Glu35 reacts with water to form hydroxyl ion, a stronger nucleophile than water, which then attacks the glycosyl enzyme intermediate, to give the product of hydrolysis and leaving the enzyme unchanged. This covalent mechanism was named after Koshland, who first came up with this type of mechanism.
More recently, quantum mechanics/ molecular mechanics (QM/MM) molecular dynamics simulations have been using the crystal of HEWL and predict the existence of a covalent intermediate. Evidence for the ESI-MS and X-ray structures indicate the existence of covalent intermediate, but primarily rely on using a less active mutant or non-native substrate. Thus, QM/MM molecular dynamics provides the unique ability to directly investigate the mechanism of wild-type HEWL and native substrate. The calculations revealed that the covalent intermediate from the Koshland mechanism is ~30 kcal/mol more stable than the ionic intermediate from the Phillips mechanism. These calculation demonstrate that the ionic intermediate is extremely energetically unfavorable and the covalent intermediates observed from experiments using less active mutant or non-native substrates provide useful insight into the mechanism of wild-type HEWL.
Inhibition
Imidazole derivatives can form a charge-transfer complex with some residues (in or outside active center) to achieve a competitive inhibition of lysozyme. In Gram-negative bacteria, the lipopolysaccharide acts as a non-competitive inhibitior by highly-favored binding with lysozyme.
Enzyme conformation changes
Lysozyme exhibits two conformations an open active state and a closed inactive state. The catalytic relevance was examined with single walled carbon nanotubes (SWCN) field effect transitors (FETs), where a singular lysozyme was bound to the SWCN FET. Electronically monitoring the lysozyme showed two conformations, an open active site and a closed inactive site. In its active state lysozyme is able to processively hydrolyze its substrate, breaking on average 100 bonds at a rate of 15 per second. In order to bind a new substrate and move from the closed inactive state to the open active state requires two conformation step changes, while inactivation requires one step.
Role in disease and therapy
Lysozyme is part of the innate immune system. Reduced lysozyme levels have been associated with bronchopulmonary dysplasia in newborns. Children pigs fed with human lysozyme milk can recover from diarrheal disease caused by E. coli faster. The concentration of lysozyme in human milk is 1,600 to 3,000 times greater than the concentration in livestock milk. Human lysozyme is more active than hen egg white lysozyme. A transgenic line of goats (with a founder named "Artemis") were developed to produce milk with human lysozyme to protect children from diarrhea if they can't get the benefits of human breastfeeding.
Since lysozyme is a natural form of protection from Gram-positive pathogens like Bacillus and Streptococcus, it plays an important rule in immunology of infants in human milk feeding. Whereas the skin is a protective barrier due to its dryness and acidity, the conjunctiva (membrane covering the eye) is, instead, protected by secreted enzymes, mainly lysozyme and defensin. However, when these protective barriers fail, conjunctivitis results.
In certain cancers (especially myelomonocytic leukemia) excessive production of lysozyme by cancer cells can lead to toxic levels of lysozyme in the blood. High lysozyme blood levels can lead to kidney failure and low blood potassium, conditions that may improve or resolve with treatment of the primary malignancy.
Serum lysozyme is much less specific for diagnosis of sarcoidosis than serum Angiotensin Converting Enzyme; however, since it is more sensitive, it is used as a marker of sarcoidosis disease activity and is suitable for disease monitoring in proven cases.
Chemical synthesis
The first chemical synthesis of a lysozyme protein was attempted by Prof. George W. Kenner and his group at the University of Liverpool in England. This was finally achieved in 2007 by Steve Kent at the University of Chicago who made synthetic functional lysozyme molecule.
Other applications
Lysozyme crystals have been used to grow other functional materials for catalysis and biomedical applications.
History
The antibacterial property of hen egg white, due to the lysozyme it contains, was first observed by Laschtschenko in 1909, although it was not until 1922 that the name 'lysozyme' was coined, by Alexander Fleming (1881–1955), the discoverer of penicillin. Fleming first observed the antibacterial action of lysozyme when he treated bacterial cultures with nasal mucus from a patient suffering from a head cold.
The three-dimensional structure of hen egg white lysozyme was described by David Chilton Phillips (1924–1999) in 1965, when he obtained the first 2-ångström (200 pm) resolution model via X-ray crystallography. The structure was publicly presented at a Royal Institution lecture in 1965. Lysozyme was the second protein structure and the first enzyme structure to be solved via X-ray diffraction methods, and the first enzyme to be fully sequenced that contains all twenty common amino acids. As a result of Phillips' elucidation of the structure of lysozyme, it was also the first enzyme to have a detailed, specific mechanism suggested for its method of catalytic action. This work led Phillips to provide an explanation for how enzymes speed up a chemical reaction in terms of its physical structures. The original mechanism proposed by Phillips was more recently revised.
