 | ||
Multiple sclerosis and other demyelinating diseases of the central nervous system (CNS) produce lesions (demyelinated areas in the CNS) and glial scars or scleroses. They present different shapes and histological findings according to the underlying condition that produces them.
Contents
- Non inflammatory demyelination
- Lesions produced by CNS Inflammatory Demyelinating diseases IDS
- Confluent vs perivenous demyelination
- ADEM demyelination
- NMO demyelination
- Confluent demyelination
- MS lesions
- Dawsons fingers
- Tumefactive demyelinating lesions
- Demyelination process in MS
- NAWM development
- BBB breakdown
- Immune mediated attack
- Lesion recovery
- Demyelination patterns in standard MS
- References
Demyelinating diseases are traditionally classified in two kinds: demyelinating myelinoclastic diseases and demyelinating leukodystrophic diseases. In the first group a normal and healthy myelin is destroyed by a toxic, chemical or autoimmune substance. In the second group, myelin is abnormal and degenerates. The second group was denominated dysmyelinating diseases by Poser Therefore, since Poser demyelinating diseases normally refers to the myelinoclastic part.
Demyelinating diseases of the CNS can be classified according to their pathogenesis into five non-exclusing categories: demyelination due to inflammatory processes, viral demyelination, demyelination caused by acquired metabolic derangements, hypoxic–ischaemic forms of demyelination and demyelination caused by focal compression.
Non inflammatory demyelination
The four non-inflammatory possibilities are:
All these four types of demyelination are non-inflammatory and different to MS even if some leukoencephalopathies can produce similar lesions
Lesions produced by CNS Inflammatory Demyelinating diseases (IDS)
Typical lesions are similar to those of MS, but there are four kinds of atypical inflammatory demyelinating lesions: Ring-like (antibody-mediated), megacystic (tumefactive), Balo-like, and diffusely-infiltrating lesions.
The list of the diseases that produce CNS demyelinating lesions is not complete, but it includes:
Confluent vs. perivenous demyelination
A special characteristic that makes a difference between MS and the several kinds of ADEM is the structure of the lesions, being strictly perivenous in ADEM and showing a confluence around veins in MS. Given that ADEM can be multiphase sometimes and MS can appear in children, this characteristic is often considered as the line that separates both conditions.
The most typical of perivenous demyelination is ADEM
ADEM demyelination
ADEM can present plaque-like lesions which are indistinguishable from MS Nevertheless, ADEM White Matter appears intact under Magnetization Transfer MRI, while MS shows problems (See NAWM). Besides ADEM does not present "black holes" under MRI (zones with axonal damage) and lesions develop strictly around veins instead of the more relaxed rule for MS
NMO demyelination
As with MS, several patterns have been described inside NMO, but they are heterogeneus inside the same individual, reflecting stages in the lesion evolution:
Early active demyelinating NMO lesions may show complement within macrophages and oligodendrocyte apoptosis associated with a selective loss of minor myelin proteins, in addition to typical NMO features in a subset of active demyelinating NMO lesions
Confluent demyelination
The demyelination around a vein is normally called "plaque". In MS plaques are reported to appear by coalescence of several confluent smaller demyelinations.
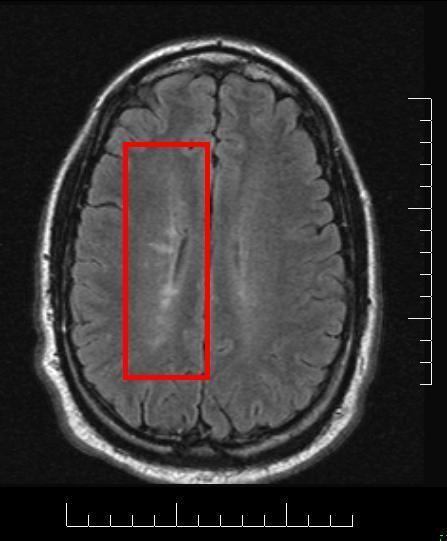
MS lesions
Normally MS lesions are small ovoid lesions, less than 2 cm. long, oriented perpendicular to the long axis of the brain's ventricles Often they are disposed surrounding a vein
Active and pre-active lesions appear as hyperintense areas under T2-weighted MRI. Pre-active lesion here refers to lesions localized in the normal appearing white matter, without apparent loss of myelin but nevertheless showing a variable degree of oedema, small clusters of microglial cells with enhanced major histocompatibility complex class II antigen, CD45 and CD68 antigen expression and a variable number of perivascular lymphocytes around small blood vessels
Using high field MRI system, with several variants several areas show lesions, and can be spacially classified in infratentorial, callosal, juxtacortical, periventricular, and other white matter areas. Other authors simplify this in three regions: intracortical, mixed gray-white matter, and juxtacortical. Others classify them as hippocampal, cortical, and WM lesions, and finally, others give seven areas: intracortical, mixed white matter-gray matter, juxtacortical, deep gray matter, periventricular white matter, deep white matter, and infratentorial lesions. The distribution of the lesions could be linked to the clinical evolution
Post-mortem authopsy reveal that gray matter demyelination occurs in the motor cortex, cingulate gyrus, cerebellum, thalamus and spinal cord. Cortical lesions have been observed specially in people with SPMS but they also appear in RRMS and clinically isolated syndrome. They are more frequent in men than in women and they can partly explain cognitive deficits.
It is known that two parameters of the cortical lesions, fractional anisotropy (FA) and mean diffusivity (MD), are higher in patients than in controls. They are larger in SPMS than in RRMS and most of them remain unchanged for short follow-up periods. They do not spread into the subcortical white matter and never show gadolinium enhancement. Over a one-year period, CLs can increase their number and size in a relevant proportion of MS patients, without spreading into the subcortical white matter or showing inflammatory features similar to those of white matter lesions.
The first plausible explanation of their distribution was published by Dr. Schelling. He said:
The specific brain plaques of multiple sclerosis can only be caused by energetic venous back-jets set in motion by intermittent rises in the pressure in the large collecting veins of the neck, but especially of the chest..But no problems with chest veins was ever found.
Recently, it has been remarked that it can plausibly be accounted for by veno-venous reflux, according to the CCSVI theory. This results in a finger-like appearance of the lesions extending mainly off the ventricles within the brain.
This morphologic appearance was named Dawson's fingers by Charles Lumsden, after the Scottish pathologist James Walker Dawson, who first defined the condition in 1916.
Dawson's fingers
"Dawson's fingers" is the name for the lesions around the ventricle-based brain veins of patients with multiple sclerosis. The condition is thought to be the result of inflammation or mechanical damage by blood pressure around long axis of medular veins.
Dawson's fingers spread along, and from, large periventricular collecting veins, and are attributed to perivenular inflammation.
Lesions far away from these veins are known as Steiner's splashes.
Sometimes experimental autoimmune encephalomyelitis has been triggered in humans by accident or medical mistake. The damage in these cases fulfils all the pathological diagnostic criteria of MS and can therefore be classified as MS in its own right. The lesions were classified as pattern II in the Lucchinetti system. This case of human EAE also showed Dawson fingers.
Tumefactive demyelinating lesions
Demyelinating lesions whose size is larger than 2 cm. They normally appear together with normal MS lesions, situation described as tumefactive multiple sclerosis. When they appear alone, they are usually named "Solitary sclerosis", being more difficult to diagnose.
They look like intracranial neoplasms, and sometimes they get biopsied as suspected tumors. Proton MR spectroscopy can help in their diagnosis.
Demyelination process in MS
The hallmark of MS is the lesion, which appears mainly in the white matter and shows macrophage mediated demyelination, BBB breakdown, inflammation and axon transection.
NAWM development
Demyelinating lesions begin with the appearance of some areas named NAWM (normal appearing white matter) which despite its name, is abnormal in several parameters. These areas show axonal transections and stressed oligodendrocytes (the cells responsible for maintaining the myelin), and randomly, they show clusters of activated microglia named pre-active lesions. These pre-lesions normally resolve themselves, though sometimes they spread towards a capilar vein.
BBB breakdown
This is followed by the blood–brain barrier (BBB) breakdown. BBB is a tight vascular barrier between the blood and brain that should prevent the passage of antibodies through it, but in MS patients it does not work. For unknown reasons special areas appear in the brain and spine, followed by leaks in the blood–brain barrier where immune cells infiltrate. This leads to the entrance of macrophages into the CNS, triggering the beginning of an immune-mediated attack against myelin. Gadolinium cannot cross a normal BBB and, therefore, Gadolinium-enhanced MRI is used to show BBB breakdowns.
Immune mediated attack
After the BBB breakdown, the immune-mediated attack against myelin happens. T cells, are a kind of lymphocyte that plays an important role in the body's defenses. The T cells recognize myelin as foreign and attack it, explaining why these cells are also called "autoreactive lymphocytes". Demyelination, further inflammation and axonal transection are the result.
The attack of myelin starts inflammatory processes, which triggers other immune cells and the release of soluble factors like cytokines and antibodies. Further breakdown of the blood–brain barrier, in turn cause a number of other damaging effects such as swelling, activation of macrophages, and more activation of cytokines and other destructive proteins.
Astrocytes can heal partially the lesion leaving a scar. These scars (sclerae) are the known plaques or lesions usually reported in MS. A repair process, called remyelination, takes place in early phases of the disease, but the oligodendrocytes are unable to completely rebuild the cell's myelin sheath. Repeated attacks lead to successively less effective remyelinations, until a scar-like plaque is built up around the damaged axons.
According to the view of most researchers, a special subset of lymphocytes, called T helper cells, specifically Th1 and Th17, play a key role in the development of the lesion. Under normal circumstances, these lymphocytes can distinguish between self and non-self. However, in a person with MS, these cells recognize healthy parts of the central nervous system as foreign and attack them as if they were an invading virus, triggering inflammatory processes and stimulating other immune cells and soluble factors like cytokines and antibodies. Many of the myelin-recognizing T cells belong to a terminally differentiated subset called co-stimulation-independent effector-memory T cells. Recently other type of immune cells, B Cells, have been also implicated in the pathogenesis of MS and in the degeneration of the axons.
The axons themselves can also be damaged by the attacks. Often, the brain is able to compensate for some of this damage, due to an ability called neuroplasticity. MS symptoms develop as the cumulative result of multiple lesions in the brain and spinal cord. This is why symptoms can vary greatly between different individuals, depending on where their lesions occur.
Lesion recovery
Under laboratory conditions, stem cells are quite capable of proliferating and differentiating into remyelinating oligodendrocytes; it is therefore suspected that inflammatory conditions or axonal damage somehow inhibit stem cell proliferation and differentiation in affected areas It is possible to predict how much and when lesion will recover
Related to this, it was found in 2016 that neural cells of primary progressive patients (PPMS) do have some kind of problem to protect neuroprotection against demyelination or oligodendrocytes, compared to healthy subjects. Some genetics seem to underly the problem as this was shown using Induced pluripotent stem cell (iPSC) as neural progenitor cells (NPC)
Demyelination patterns in standard MS
Four different damage patterns, known as Lassmann patterns, have been identified by her team in the scars of the brain tissue in multiple sclerosis, and they are used sometimes as a basis for describing lesions in other demyelinating diseases.
The meaning of this fact is controversial. For some investigation teams it means that MS is a heterogeneous disease. Others maintain that the shape of the scars can change with time from one type to other and this could be a marker of the disease evolution. Anyway, the heterogeneity could be true only for the early stage of the disease. Some lesions present mitochondrial defects that could distinguish types of lesions. Currently antibodies to lipids and peptides in sera, detected by microarrays, can be used as markers of the pathological subtype given by brain biopsy.
After some debate among research groups, currently the heterogeneity hypothesis looks like accepted
