Formula HClO | Molar mass 52.46 g/mol | |
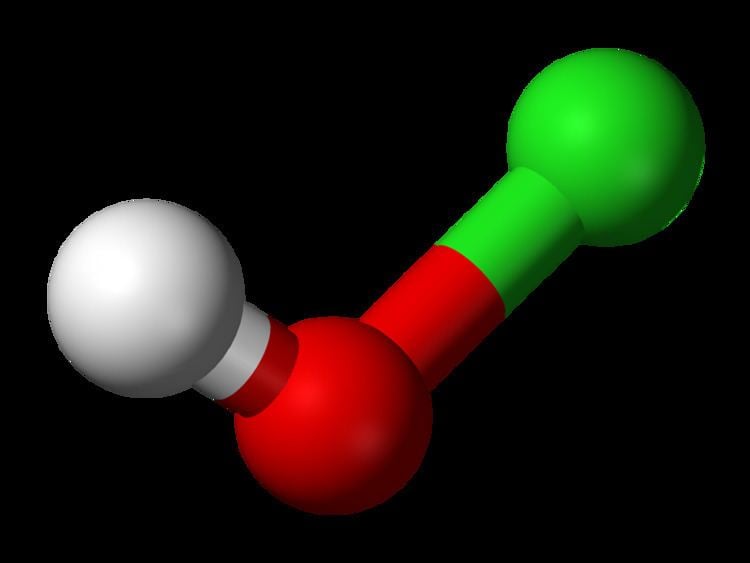 | ||
Related compounds Appearance Colorless aqueous solution | ||
Dr michael explains electrolyzed hypochlorous acid
Hypochlorous acid (HClO) is a weak acid that forms when chlorine dissolves in water, and itself partially dissociates, forming ClO-. HClO and ClO- are oxidizers, and the primary disinfection agents of chlorine solutions. HClO cannot be isolated from these solutions due to rapid equilibration with its precursor. Sodium hypochlorite (NaClO) and calcium hypochlorite (Ca(ClO)2), are bleaches, deodorants, and disinfectants.
Contents
- Dr michael explains electrolyzed hypochlorous acid
- About hypochlorous acid
- Uses
- Formation stability and reactions
- Chemical reactions
- Reactivity of HClO with biomolecules
- Reaction with protein sulfhydryl groups
- Reaction with protein amino groups
- Reaction with DNA and nucleotides
- Reaction with lipids
- Mode of disinfectant action
- Inhibition of glucose oxidation
- Depletion of adenine nucleotides
- Inhibition of DNA replication
- Protein unfolding and aggregation
- Hypochlorites
- Production of hypochlorites using electrolysis
- Safety
- Uses of Hypochlorous Acid
- References
About hypochlorous acid
Uses
In organic synthesis, HClO converts alkenes to chlorohydrins.
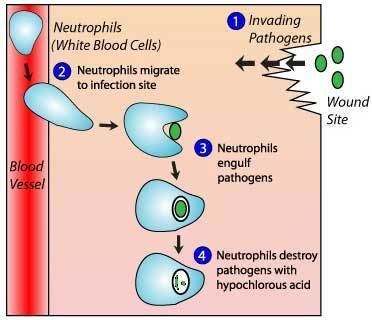
In biology, hypochlorous acid is generated in activated neutrophils by myeloperoxidase-mediated peroxidation of chloride ions, and contributes to the destruction of bacteria.
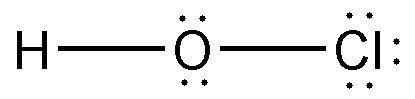
In the cosmetics industry it is used as a skin cleansing agent, which benefits the body's skin rather than causing drying. It is also used in baby products, because baby skin is particularly sensitive and can be easily irritated.
In water treatment, hypochlorous acid is the active sanitizer in hypochlorite-based products (e.g. used in swimming pools).
In food service and water distribution, specialized equipment to generate weak solutions of HClO from water and salt is sometimes used to generate adequate quantities of safe (unstable) disinfectant to treat food preparation surfaces and water supplies.
Formation, stability and reactions
Addition of chlorine to water gives both hydrochloric acid (HCl) and hypochlorous acid (HClO):
Cl2 + H+2O ⇌ HClO + HClCl2 + 4 OH− ⇌ 2 ClO− + 2 H2O + 2 e−Cl2 + 2 e− ⇌ 2 Cl−When acids are added to aqueous Salts of hypochlorous acid (such as sodium hypochlorite in commercial bleach solution), the resultant reaction is driven to the left, and chlorine gas is formed. Thus, the formation of stable hypochlorite bleaches is facilitated by dissolving chlorine gas into basic water solutions, such as sodium hydroxide.
The acid can also be prepared by dissolving dichlorine monoxide in water; under standard aqueous conditions, anhydrous hypochlorous acid is impossible to prepare due to the readily reversible equilibrium between it and its anhydride:
2 HOCl ⇌ Cl2O + H2O K (at 0 °C) = 3.55×10−3 dm3 mol−1The presence of light or transition metal oxides of copper, nickel, or cobalt accelerates the exothermic decomposition into hydrochloric acid and oxygen:
2 Cl2 + 2 H2O → 4 HCl + O2Chemical reactions
In aqueous solution, hypochlorous acid partially dissociates into the anion hypochlorite ClO−:
HClO ⇌ ClO− + H+Salts of hypochlorous acid are called hypochlorites. One of the best-known hypochlorites is NaClO, the active ingredient in bleach.
HClO is a stronger oxidant than chlorine under standard conditions.
2 HClO(aq) + 2 H+ + 2 e− ⇌ Cl2(g) + 2 H2O E = +1.63 VHClO reacts with HCl to form chlorine gas:
HClO + HCl → H2O + Cl2HClO reacts with amines to form chloramines and water. Reacting with ammonia:
NH3 + HClO → NH2Cl + H2OHClO can also react with organic amines, forming N-chloroamines.
Reactivity of HClO with biomolecules
Hypochlorous acid reacts with a wide variety of biomolecules, including DNA, RNA, fatty acid groups, Cholesterol and proteins.
Reaction with protein sulfhydryl groups
Knox et al. first noted that HClO is a sulfhydryl inhibitor that, in sufficient quantity, could completely inactivate proteins containing sulfhydryl groups. This is because HClO oxidises sulfhydryl groups, leading to the formation of disulfide bonds that can result in crosslinking of proteins. The HClO mechanism of sulfhydryl oxidation is similar to that of chloramine, and may only be bacteriostatic, because once the residual chlorine is dissipated, some sulfhydryl function can be restored. One sulfhydryl-containing amino acid can scavenge up to four molecules of HOCl. Consistent with this, it has been proposed that sulfhydryl groups of sulfur-containing amino acids can be oxidized a total of three times by three HClO molecules, with the fourth reacting with the α-amino group. The first reaction yields sulfenic acid (R–SOH) then sulfinic acid (R–SO2H) and finally R–SO3H. Sulfenic acids form disulfides with another protein sulfhydryl group, causing cross-linking and aggregation of proteins. Sulfinic acid and R–SO3H derivatives are produced only at high molar excesses of HClO, and disulfides are formed primarily at bacteriocidal levels. Disulfide bonds can also be oxidized by HClO to sulfinic acid. Because the oxidation of sulfhydryls and disulfides evolves hydrochloric acid, this process results in the depletion HClO.
Reaction with protein amino groups
Hypochlorous acid reacts readily with amino acids that have amino group side-chains, with the chlorine from HClO displacing a hydrogen, resulting in an organic chloramine. Chlorinated amino acids rapidly decompose, but protein chloramines are longer-lived and retain some oxidative capacity. Thomas et al. concluded from their results that most organic chloramines decayed by internal rearrangement and that fewer available NH2 groups promoted attack on the peptide bond, resulting in cleavage of the protein. McKenna and Davies found that 10 mM or greater HClO is necessary to fragment proteins in vivo. Consistent with these results, it was later proposed that the chloramine undergoes a molecular rearrangement, releasing HCl and ammonia to form an amide. The amide group can further react with another amino group to form a Schiff base, causing cross-linking and aggregation of proteins.
Reaction with DNA and nucleotides
Hypochlorous acid reacts slowly with DNA and RNA as well as all nucleotides in vitro. GMP is the most reactive because HClO reacts with both the heterocyclic NH group and the amino group. In similar manner, TMP with only a heterocyclic NH group that is reactive with HClO is the second-most reactive. AMP and CMP, which have only a slowly reactive amino group, are less reactive with HClO. UMP has been reported to be reactive only at a very slow rate. The heterocyclic NH groups are more reactive than amino groups, and their secondary chloramines are able to donate the chlorine. These reactions likely interfere with DNA base pairing, and, consistent with this, Prütz has reported a decrease in viscosity of DNA exposed to HClO similar to that seen with heat denaturation. The sugar moieties are nonreactive and the DNA backbone is not broken. NADH can react with chlorinated TMP and UMP as well as HClO. This reaction can regenerate UMP and TMP and results in the 5-hydroxy derivative of NADH. The reaction with TMP or UMP is slowly reversible to regenerate HClO. A second slower reaction that results in cleavage of the pyridine ring occurs when excess HClO is present. NAD+ is inert to HClO.
Reaction with lipids
Hypochlorous acid reacts with unsaturated bonds in lipids, but not saturated bonds, and the ClO− ion does not participate in this reaction. This reaction occurs by hydrolysis with addition of chlorine to one of the carbons and a hydroxyl to the other. The resulting compound is a chlorhydrin. The polar chlorine disrupts lipid bilayers and could increase permeability. When chlorhydrin formation occurs in lipid bilayers of red blood cells, increased permeability occurs. Disruption could occur if enough chlorhydrin is formed. The addition of preformed chlorhydrins to red blood cells can affect permeability as well. Cholesterol chlorhydrins have also been observed, but do not greatly affect permeability, and it is believed that Cl2 is responsible for this reaction.
Mode of disinfectant action
Escherichia coli exposed to hypochlorous acid lose viability in less than 0.1 seconds due to inactivation of many vital systems. Hypochlorous acid has a reported LD50 of 0.0104–0.156 ppm and 2.6 ppm caused 100% growth inhibition in 5 minutes. However it should be noted that the concentration required for bactericidal activity is also highly dependent on bacterial concentration.
Inhibition of glucose oxidation
In 1948, Knox et al. proposed the idea that inhibition of glucose oxidation is a major factor in the bacteriocidal nature of chlorine solutions. He proposed that the active agent or agents diffuse across the cytoplasmic membrane to inactivate key sulfhydryl-containing enzymes in the glycolytic pathway. This group was also the first to note that chlorine solutions (HOCl) inhibit sulfhydryl enzymes. Later studies have shown that, at bacteriocidal levels, the cytosol components do not react with HOCl. In agreement with this, McFeters and Camper found that aldolase, an enzyme that Knox et al. proposes would be inactivated, was unaffected by HOCl in vivo. It has been further shown that loss of sulfhydryls does not correlate with inactivation. That leaves the question concerning what causes inhibition of glucose oxidation. The discovery that HOCl blocks induction of β-galactosidase by added lactose led to a possible answer to this question. The uptake of radiolabeled substrates by both ATP hydrolysis and proton co-transport may be blocked by exposure to HOCl preceding loss of viability. From this observation, it proposed that HOCl blocks uptake of nutrients by inactivating transport proteins. The question of loss of glucose oxidation has been further explored in terms of loss of respiration. Venkobachar et al. found that succinic dehydrogenase was inhibited in vitro by HOCl, which led to the investigation of the possibility that disruption of electron transport could be the cause of bacterial inactivation. Albrich et al. subsequently found that HOCl destroys cytochromes and iron-sulfur clusters and observed that oxygen uptake is abolished by HOCl and adenine nucleotides are lost. It was also observed that irreversible oxidation of cytochromes paralleled the loss of respiratory activity. One way of addressing the loss of oxygen uptake was by studying the effects of HOCl on succinate-dependent electron transport. Rosen et al. found that levels of reductable cytochromes in HOCl-treated cells were normal, and these cells were unable to reduce them. Succinate dehydrogenase was also inhibited by HOCl, stopping the flow of electrons to oxygen. Later studies revealed that Ubiquinol oxidase activity ceases first, and the still-active cytochromes reduce the remaining quinone. The cytochromes then pass the electrons to oxygen, which explains why the cytochromes cannot be reoxidized, as observed by Rosen et al. However, this line of inquiry was ended when Albrich et al. found that cellular inactivation precedes loss of respiration by using a flow mixing system that allowed evaluation of viability on much smaller time scales. This group found that cells capable of respiring could not divide after exposure to HOCl.
Depletion of adenine nucleotides
Having eliminated loss of respiration, Albrich et al. proposes that the cause of death may be due to metabolic dysfunction caused by depletion of adenine nucleotides. Barrette et al. studied the loss of adenine nucleotides by studying the energy charge of HOCl-exposed cells and found that cells exposed to HOCl were unable to step up their energy charge after addition of nutrients. The conclusion was that exposed cells have lost the ability to regulate their adenylate pool, based on the fact that metabolite uptake was only 45% deficient after exposure to HOCl and the observation that HOCl causes intracellular ATP hydrolysis. It was also confirmed that, at bacteriocidal levels of HOCl, cytosolic components are unaffected. So it was proposed that modification of some membrane-bound protein results in extensive ATP hydrolysis, and this, coupled with the cells inability to remove AMP from the cytosol, depresses metabolic function. One protein involved in loss of ability to regenerate ATP has been found to be ATP synthetase. Much of this research on respiration reconfirms the observation that relevant bacteriocidal reactions take place at the cell membrane.
Inhibition of DNA replication
Recently it has been proposed that bacterial inactivation by HOCl is the result of inhibition of DNA replication. When bacteria are exposed to HOCl, there is a precipitous decline in DNA synthesis that precedes inhibition of protein synthesis, and closely parallels loss of viability. During bacterial genome replication, the origin of replication (oriC in E. coli) binds to proteins that are associated with the cell membrane, and it was observed that HOCl treatment decreases the affinity of extracted membranes for oriC, and this decreased affinity also parallels loss of viability. A study by Rosen et al. compared the rate of HOCl inhibition of DNA replication of plasmids with different replication origins and found that certain plasmids exhibited a delay in the inhibition of replication when compared to plasmids containing oriC. Rosen’s group proposed that inactivation of membrane proteins involved in DNA replication are the mechanism of action of HOCl.
Protein unfolding and aggregation
HOCl is known to cause post-translational modifications to proteins, the notable ones being cysteine and methionine oxidation. A recent examination of HOCl's bactericidal role revealed it to be a potent inducer of protein aggregation. Hsp33, a chaperone known to be activated by oxidative heat stress, protects bacteria from the effects of HOCl by acting as a holdase, effectively preventing protein aggregation. Strains of E. coli and Vibrio cholerae lacking Hsp33 were rendered especially sensitive to HOCl. Hsp33 protected many essential proteins from aggregation and inactivation due to HOCl, which is a probable mediator of HOCl's bactericidal effects.
Hypochlorites
Hypochlorites are the salts of hypochlorous acid; commercially important hypochlorites are calcium hypochlorite and sodium hypochlorite.
Production of hypochlorites using electrolysis
Solutions of hypochlorites can be produced by electrolysis of an aqueous chloride solution. The composition of the resulting solution depends on the pH at the anode. In acid conditions the solution produced will have a high hypochlorous acid concentration, but will also contain dissolved gaseous chlorine, which can be corrosive, at a neutral pH the solution will be around 75% hypochlorous acid and 25% hypochlorite. Some of the chlorine gas produced will dissolve forming hypochlorite ions. Hypochlorites are also produced by the disproportionation of chlorine gas in alkaline solutions.
Safety
HOCl is a strong oxidising agent.
Uses of Hypochlorous Acid
Hypochlorous acid has been investigated as a possible wound care agent, and as of early 2016 the U.S. Food and Drug Administration has approved products whose main active ingredient is hypochlorous acid for use in treating wounds and various infections in humans and pets. It is also FDA-approved as a preservative for saline solutions.
