 | ||
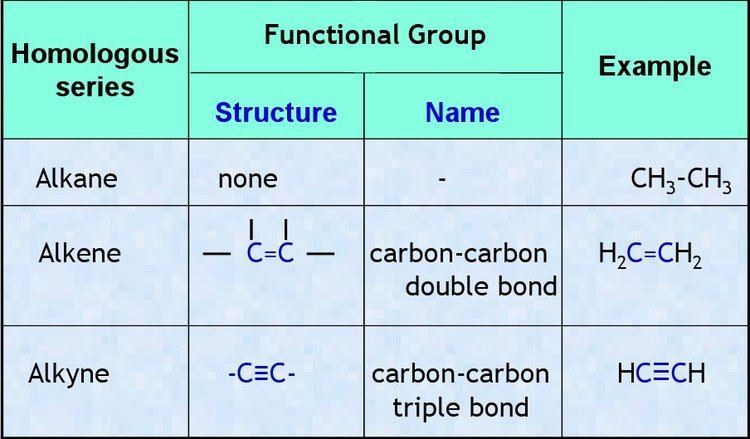
In chemistry, a homologous series is a series of compounds with the same general formula, usually varying by a single parameter—such as the length of a carbon chain. Examples of such series are the straight-chained alkanes (paraffins), and some of their derivatives (such as the primary alcohols, aldehydes, and (mono)carboxylic acid). The single-ring unbranched cycloalkanes form another such series.
Contents
- Carbon its compounds lecture 20 class x homologous series chemistry
- Example straight chained alkanes
- Inorganic homologous series
- References
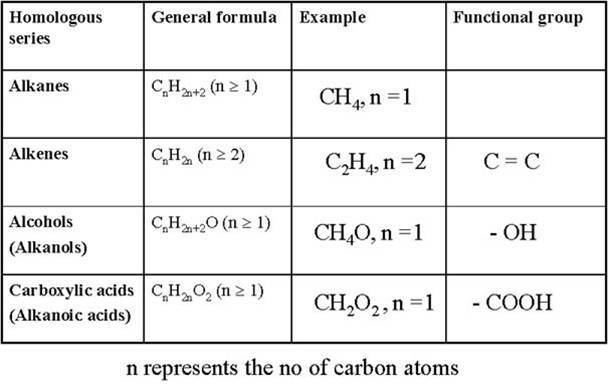
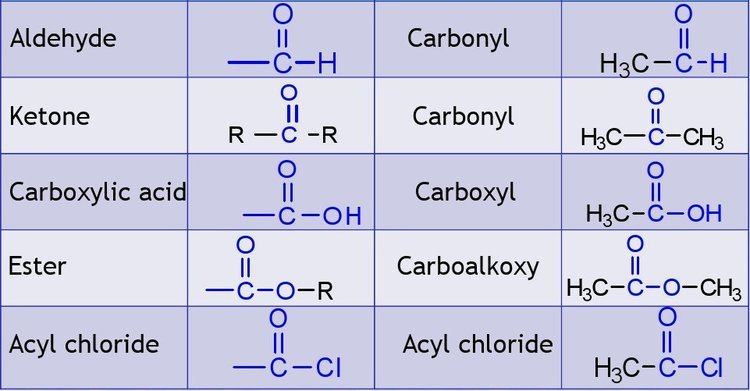
Compounds within a homologous series typically have a fixed set of functional groups that gives them similar chemical and physical properties. (For example, the series of primary straight-chained alcohols has an hydroxyl at the end of the carbon chain.) These properties typically change gradually along the series, and the changes can often be explained by mere differences in molecular size and mass. The name "homologous series" is also often used for any collection of compounds that have similar structures or include the same functional group, such as the general alkanes (straight and branched), the alkenes (olefins), the carbohydrates, etc. However, if the members cannot be arranged in a linear order by a single parameter, the collection may be better called a "chemical family" or "class of homologous compounds" than a "series".
The concept of homologous series was proposed in 1843 by the French chemist Charles Gerhardt.
Carbon its compounds lecture 20 class x homologous series chemistry
Example: straight-chained alkanes
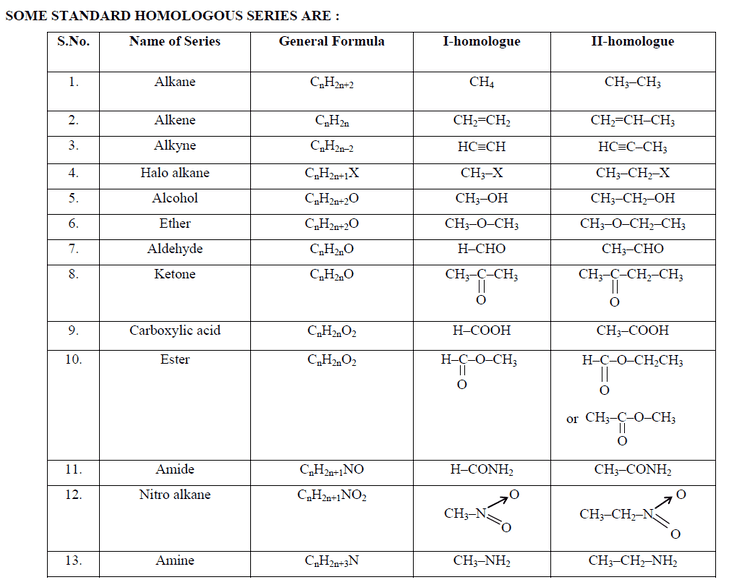
The homologous series of straight-chained alkanes begins methane (CH4), ethane (C2H6), propane (C3H8), butane (C4H10), and pentane (C5H12). In that series, successive members differ in mass by an extra methylene bridge (-CH2- unit) inserted in the chain. Thus the molecular mass of each member differs by 14 atomic mass units. Adjacent members in such a series, such as methane and ethane, are known as "adjacent homologues."
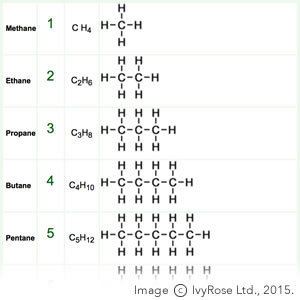
Within that series, many physical properties such as boiling point gradually increase with increasing mass. For example, ethane (C2H6), has a higher boiling point than methane (CH4). This is because an ethane molecule experiences greater dipole moments, as in a large molecule, the electron cloud tends to distort at random to a greater extent. Thus, the London dispersion forces between ethane molecules are higher than that between methane molecules, resulting in stronger forces of intermolecular attraction, raising the boiling point.
The corresponding homologous series of primary straight-chained alcohol comprises methanol (CH4O), ethanol (C2H6O), 1-propanol (C3H8O), 1-butanol, and so on.
A homologation reaction is a chemical process that converts one member of a homologous series to the next member.
Inorganic homologous series
Homologous series are not unique to organic chemistry. Titanium, vanadium, and molybdenum oxides all form homologous series (e.g. VnO2n − 1 for 2 < n < 10), as do the silanes, SinH2n + 2 (with n up to 8) that are analogous to the alkanes, CnH2n + 2.
